
Cardiac inotropy refers to the contractile strength of heart muscle. Positive inotropes strengthen the force of cardiac muscle contractions, and negative inotropes weaken them. Both positive and negative inotropes are used in the management of various cardiovascular conditions. Cardiomyocytes cultured in MEA plates form a spontaneously beating syncytium. As the cardiomyocytes mechanically contract and relax over a recording electrode, the shape change is detected as an increase and decrease in the impedance signal.
The Maestro Pro and Edge systems detect key parameters of cardiomyocyte contractility, including beat amplitude, beat timing, and excitation-contraction delay. Maestro Pro & Edge are the only platforms that allow measurement of cardiomyocyte electrical activity and the resulting contraction on the same electrode, providing a more complete understanding of your cardiac model's functionality.
-
Elicit mature force-frequency response>
-
Detect inotropic compounds>
-
Monitor changes in contractility for 3D cultures>
-
Assay Steps>
Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) are limited by functional immaturity, including immature calcium handling and contractility function. Physical conditioning via electrical or mechanical stimulation facilitates cardiomyocyte maturation as measured by a positive force-frequency relationship.
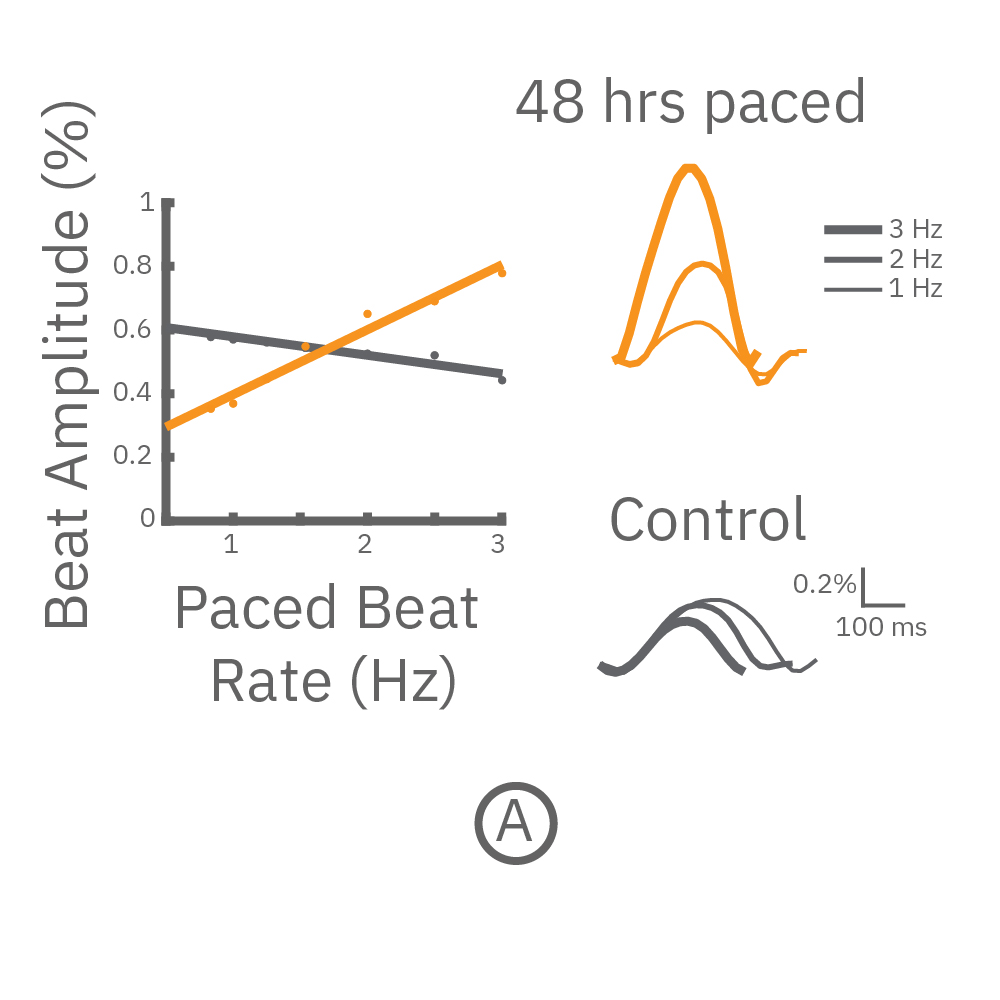
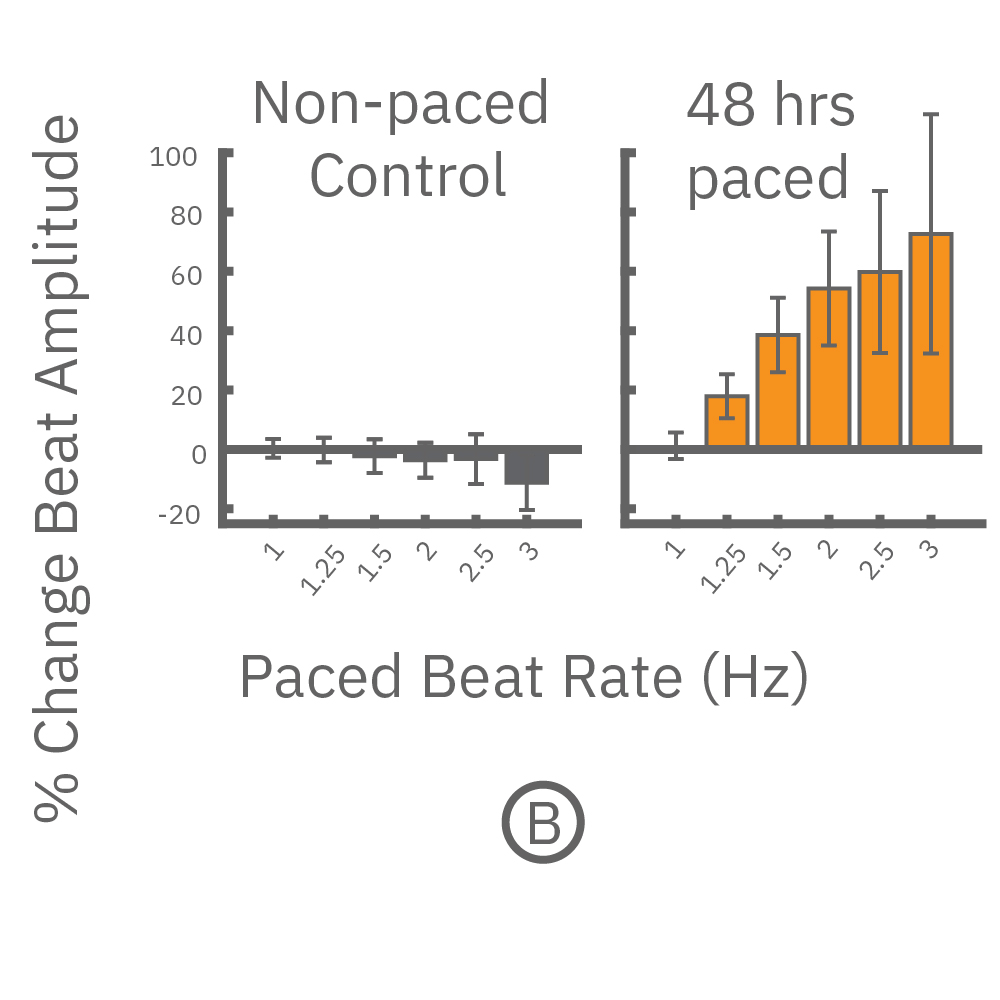
(A,B) FCDI iCell cardiomyocytes2 (CM2 ) were chronically paced at 2 Hz for 48 hours. (A,B) After chronic pacing, cells were paced at 0.8 up to 3 Hz to assess the force-frequency relationship. Paced wells (orange) exhibited a positive force-frequency relationship after only 48 hours, while non-paced control wells (gray) showed a slight negative relationship. Representative paced (orange) and control (gray) contractility traces are shown at 1 Hz (thin line), 2 Hz, and 3 Hz (thick line).
The immature phenotype of hiPSC-cardiomyocytes is a well-recognized limitation in inotropic evaluation, in particular, the lack of or diminished positive inotropic response to β-adrenergic receptor agonists. Chronic pacing of hiPSC-cardiomyocytes at 2 Hz for 48 hours on the Maestro MEA system, facilitates the detection of positive inotropes.
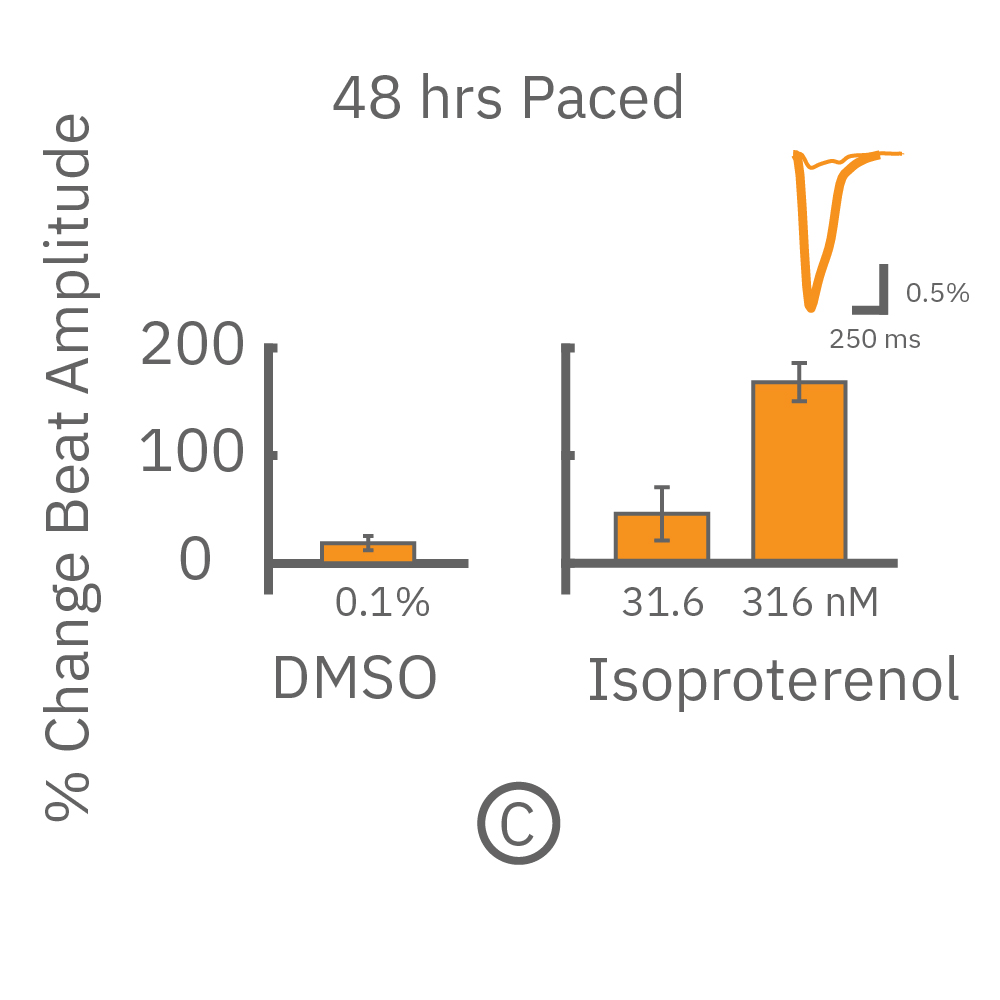
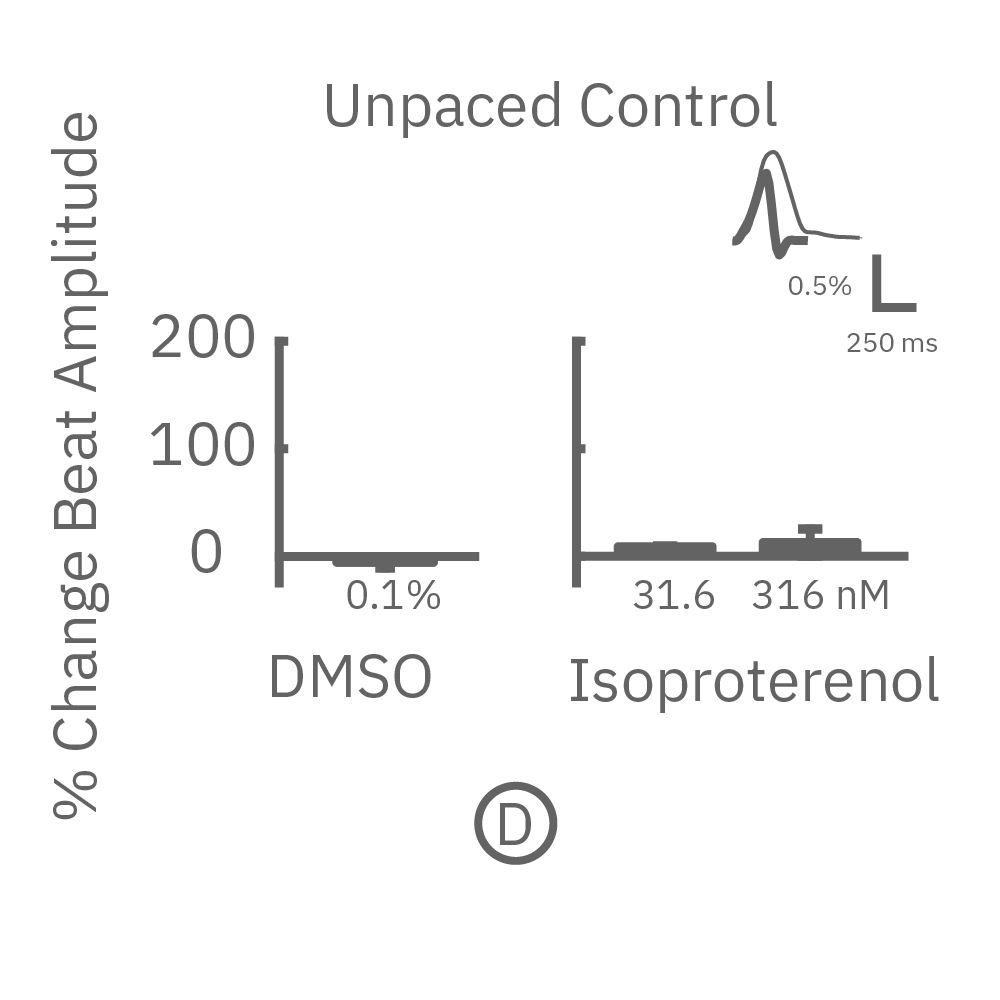
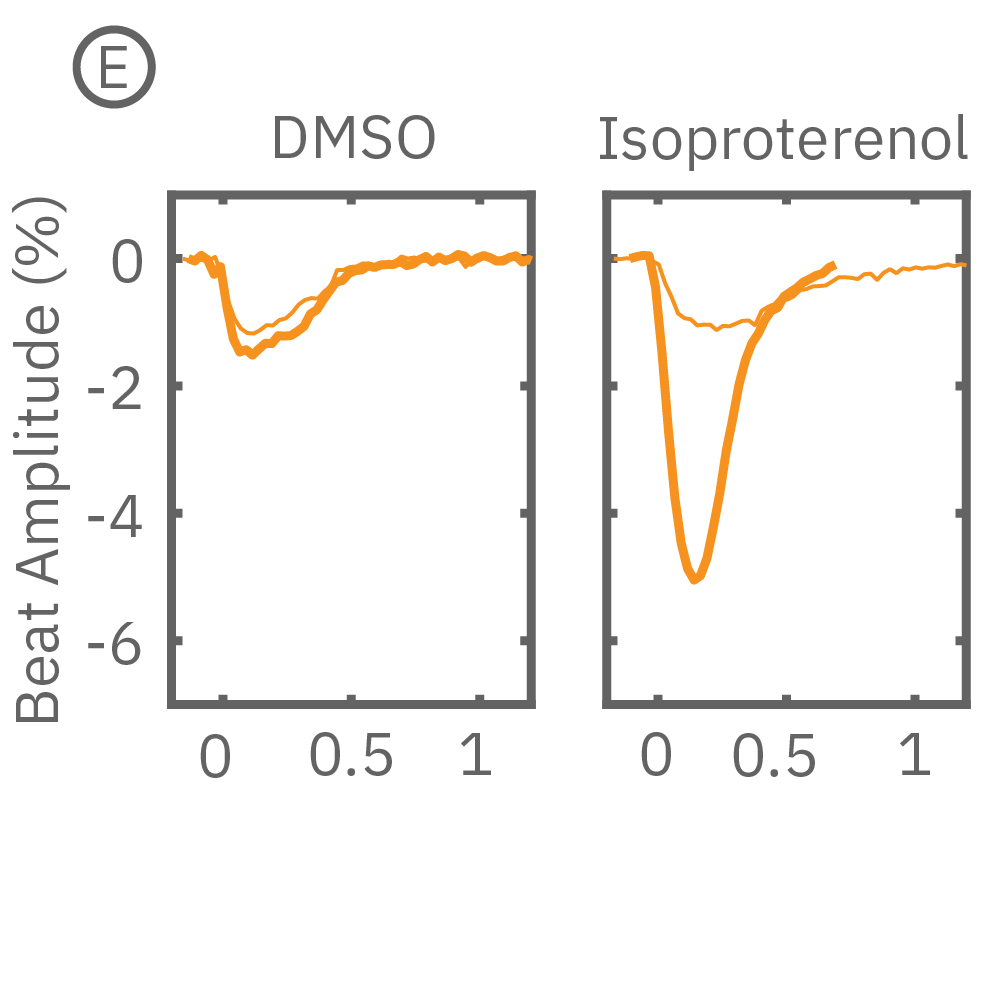
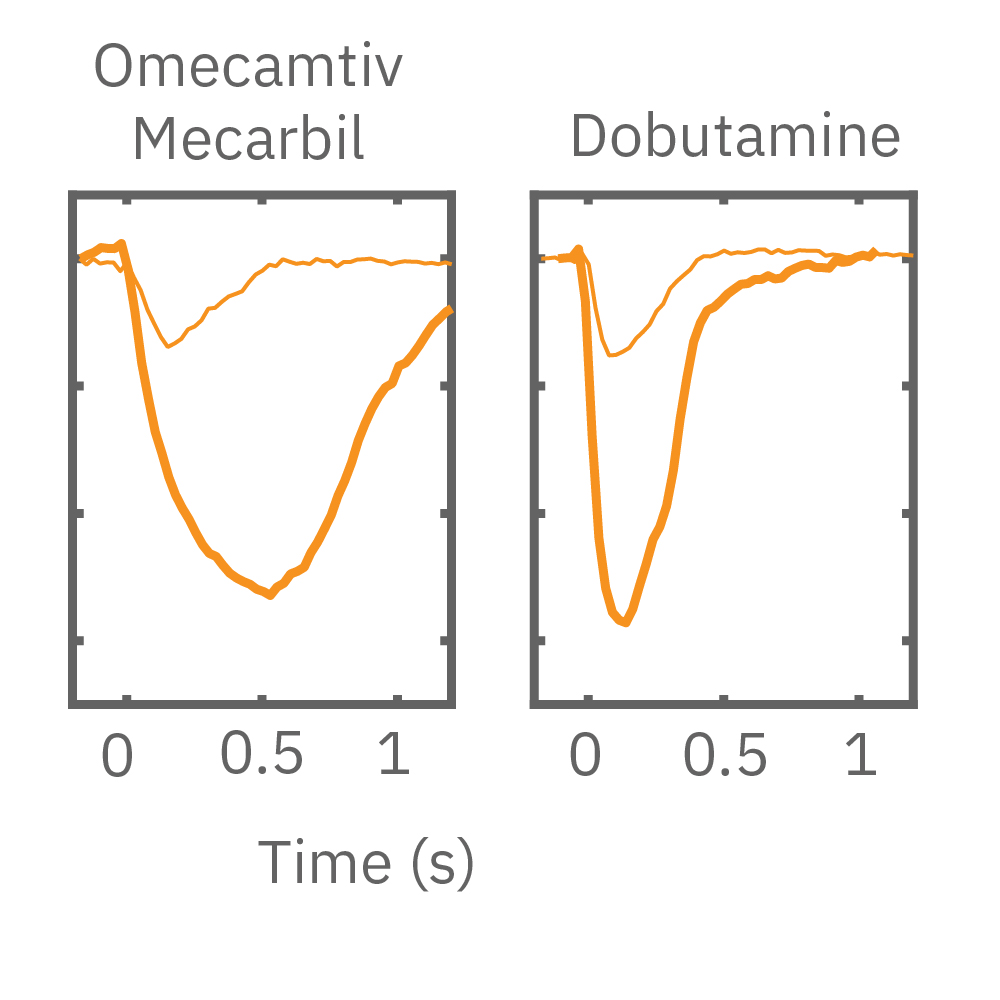
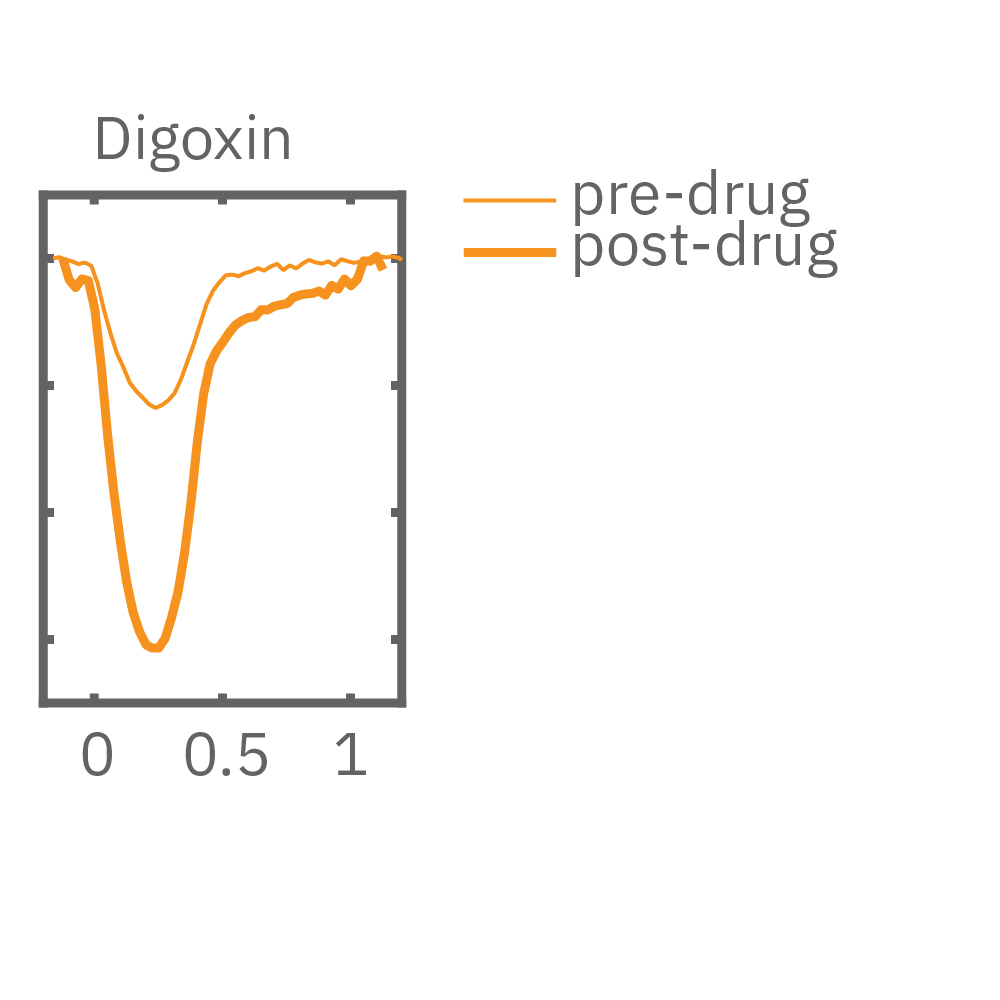
After chronic pacing at 2 Hz for 48 hours, FCDI iCell CM2 were dosed with positive inotropes, such as isoproterenol. Chronically paced wells (C, orange) showed a dose-dependent increase in beat amplitude in response to isoproterenol (a β-adrenergic receptor agonist), while non-paced control wells (D, gray) showed no response. (E) Similarly, chronically paced wells also successfully detected a variety of other positive inotropes. Beat amplitude is calculated as the % change of total impedance.
Three-dimensional in vitro cell models, such as spheroids and organoids, more accurately recapitulate the multicellular organization and structure of in vivo tissues, making them powerful for disease modeling, developmental biology, and drug safety. Recording from an array of microelectrodes enables contractility measures from one or more 3D cardiac spheroids in a well.
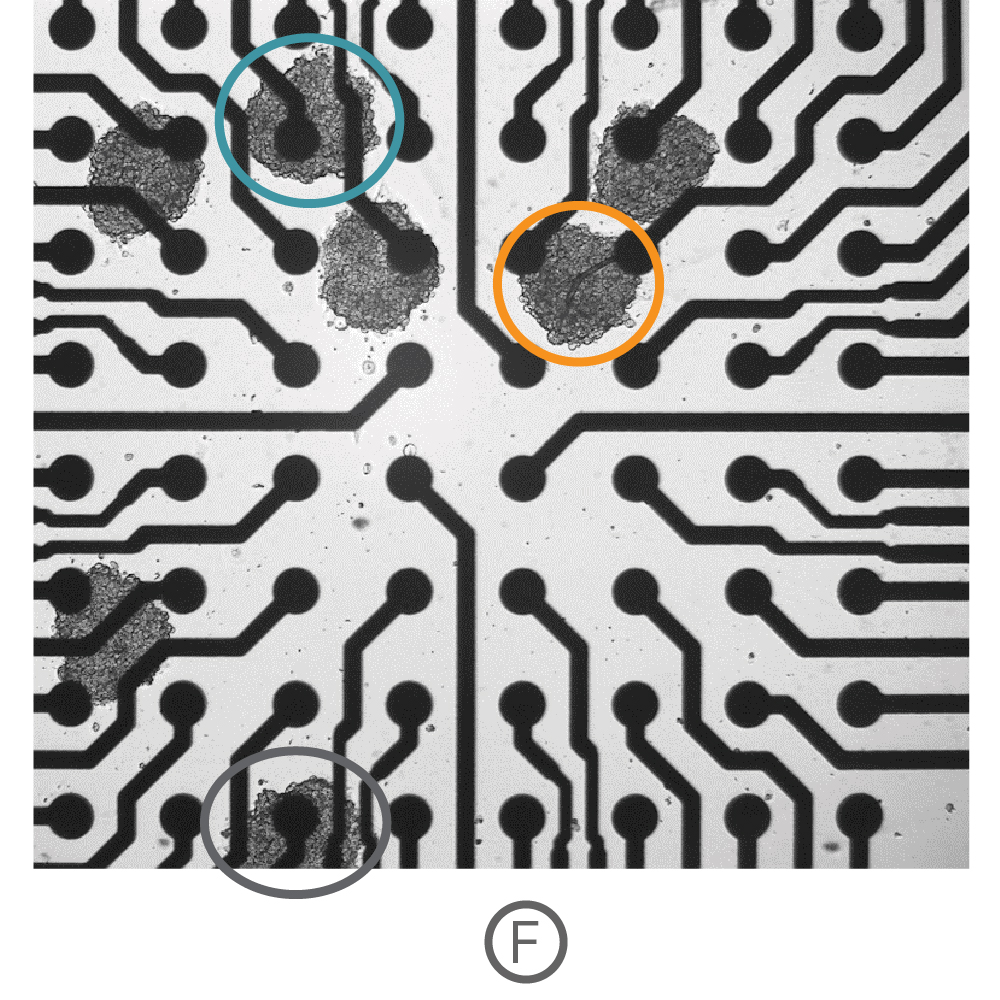
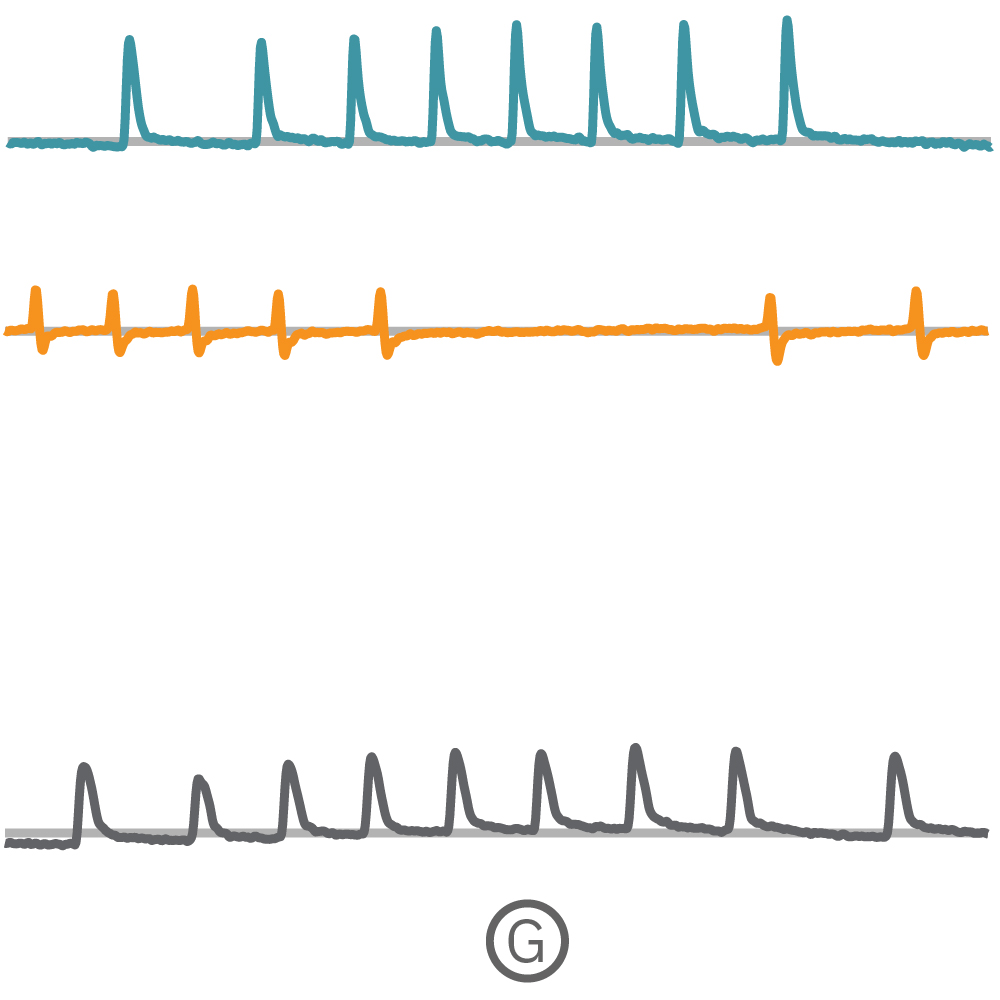
(F) Multiple Ncardia Cor.4U spheroids were deposited in each well of a CytoView MEA 6-well plate. (G) As the spheroids attached, multiple independently beating cultures were readily distinguished using array-based contractility. Each circled spheroid culture (F) corresponds to a single contractility trace (G).
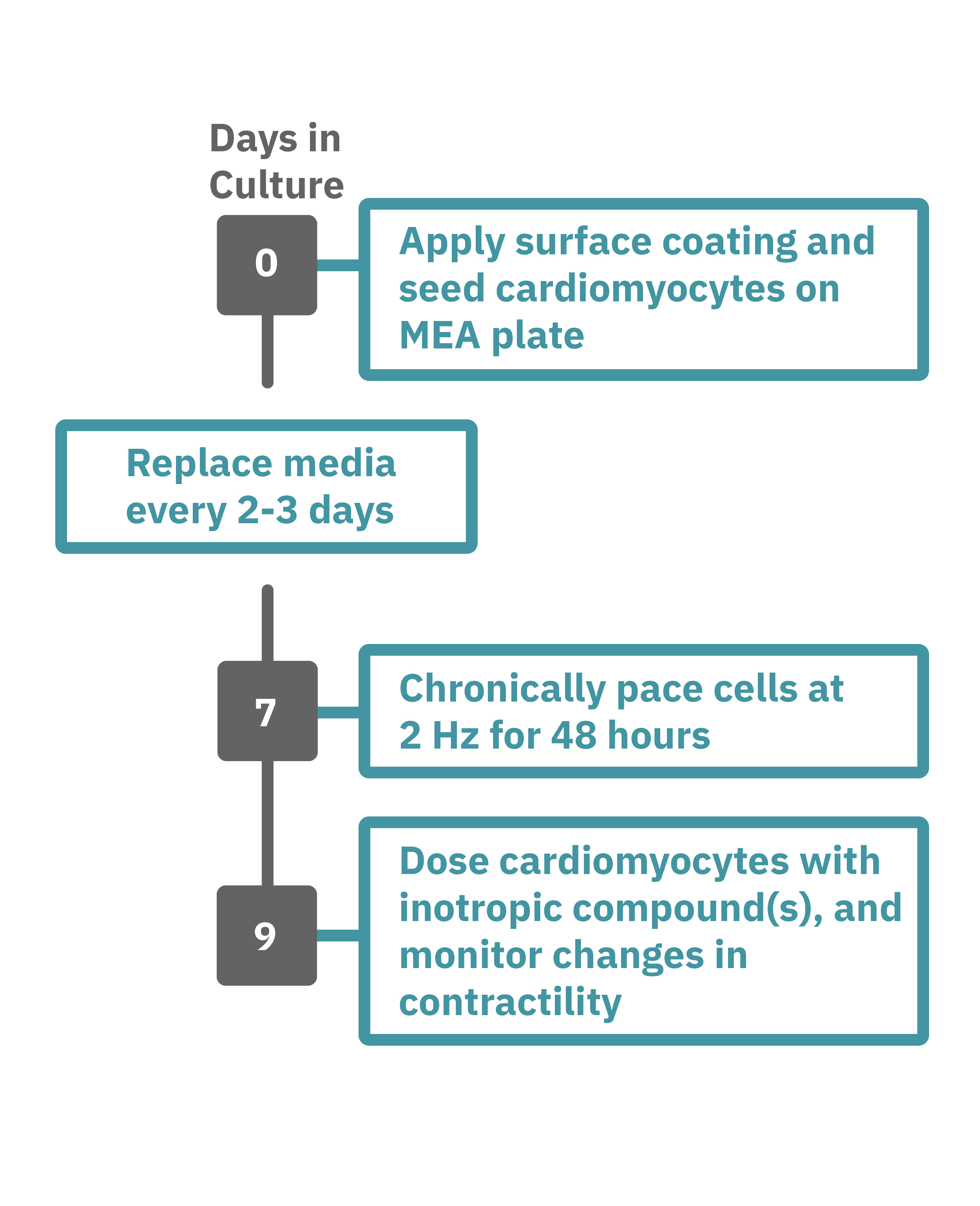
Getting started with Maestro Edge or Pro couldn't be easier. Culture your cells in an Axion multiwell plate (Day 0). Load this plate into the Maestro system and allow the environmental chamber to automatically equilibrate. The array of electrodes can detect regions of the cell culture that are contracting while other regions are being stretched. Chronically pace the cardiomyocytes at 2 Hz for 48 hours on the Maestro MEA system (Day 7). Analyze changes in contractility of your cardiomyocytes label-free and in real-time with AxIS Navigator software (Day 9).





