What are the advantages of MEA to study in vitro epilepsy disease models?
Epilepsy is a neurological disorder characterized by reoccuring seizures, often caused by abnormal electrical activity in the brain. Understanding the alterations in neural networks is crucial to unraveling the fundamental triggers and mechanisms behind epileptic seizures.
Axion’s live-cell assay platforms offer an ideal approach for investigating epilepsy and its effects on neural networks. Noninvasive, real-time analysis preserves the intricate connections and signaling within neural circuits, enabling researchers to observe and study the progression of epilepsy in vitro.
Understanding mechanisms of epilepsy and seizures with in vitro models
Studying epilepsy requires innovative lab tools designed to accurately measure complex neural network function.
The Maestro MEA platform can recreate in vivo phenotypes of seizurogenic activity in vitro, allowing researchers to:
- Compare patient lines side-by-side
- Screen therapeutics and assess functional impacts
- Monitor and evaluate cultures over time and label-free
Discover how Axion’s live-cell analysis tools can reveal the mechanisms of epilepsy.
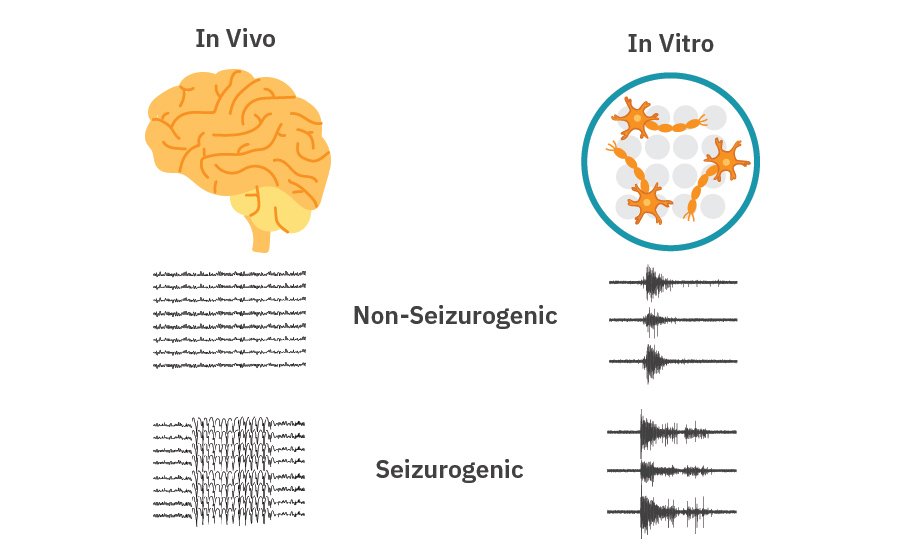
Exploring neural activity with in vitro models of epilepsy and seizures
-
Modulation of neuronal network activity to understand the mechanisms of epilepsy>
-
Studying pediatric epilepsy with stem cell-based models>
-
In vitro model to assess of the role of isocitrate dehydrogenase mutation in the development of glioblastoma-induced seizures>
-
AI-based MEA assay assesses drug-induced neurotoxicity and seizure risk in vitro>
-
Publication Highlights: Epilepsy>
Purpose: To understand the effects of microRNA 128 on the excitability of neuronal networks. Recent studies have implicated the involvement of certain microRNAs in development of epilepsy.
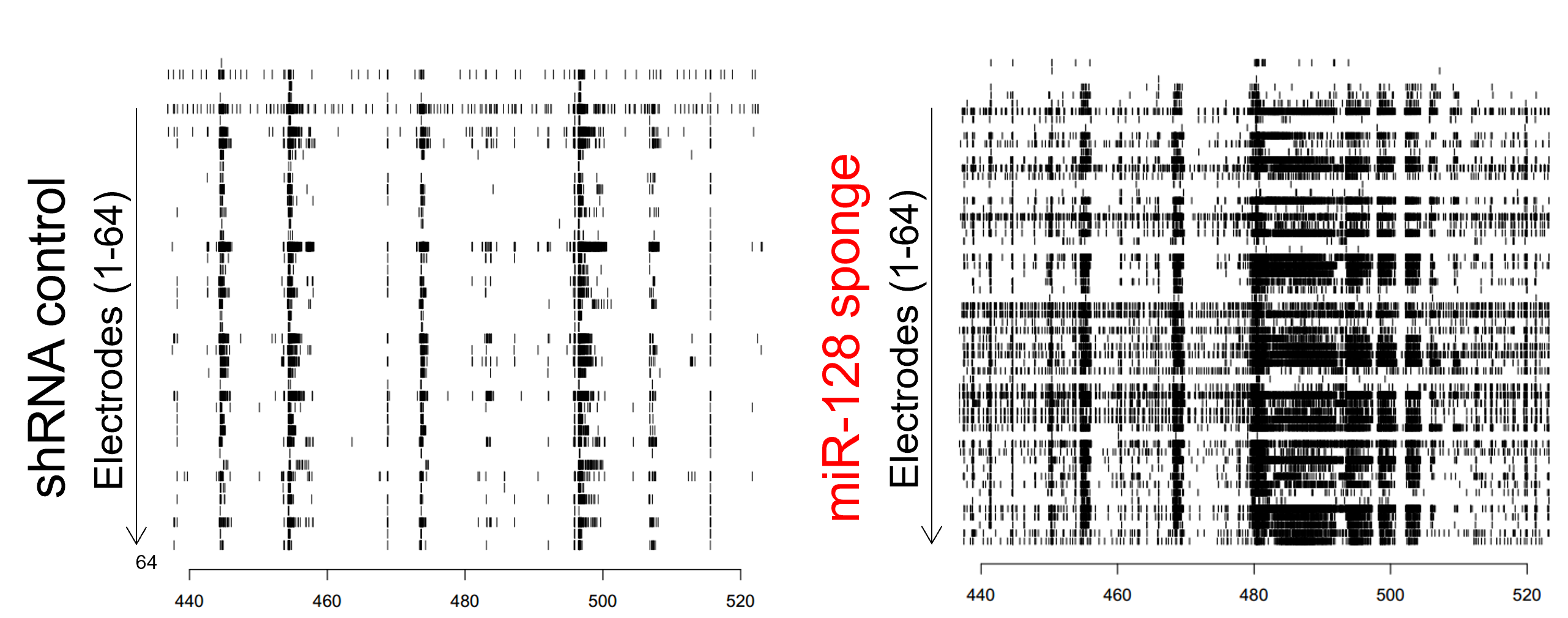
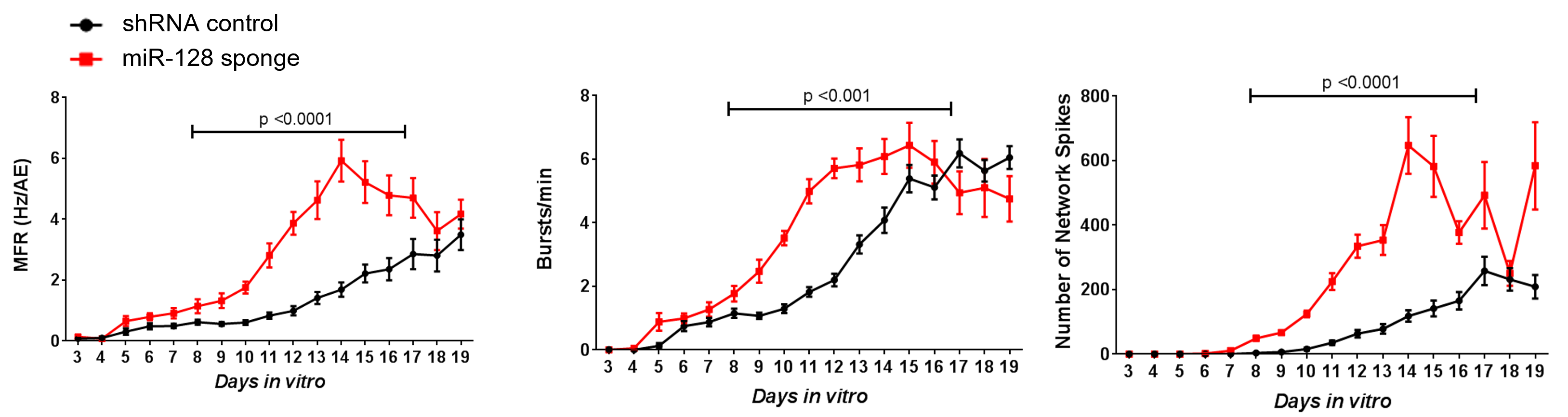
The network excitability of primary mouse cortical neurons, with suppressed expression of microRNA 128, was studied on the Maestro MEA platform.
Result: The Maestro MEA data indicated that neurons with microRNA 128 knockdown expression exhibited increased overall firing, bursting, and number of network spikes, suggesting microRNA 128 suppression induces network hyperexcitability phenotype [McSweeney 2016].
Purpose: To test induced pluripotent stem cell (iPSC)-based model and establish its suitability for the study of pediatric epilepsy. The lack of appropriate models represents the primary challenge in the fight against epilepsy.
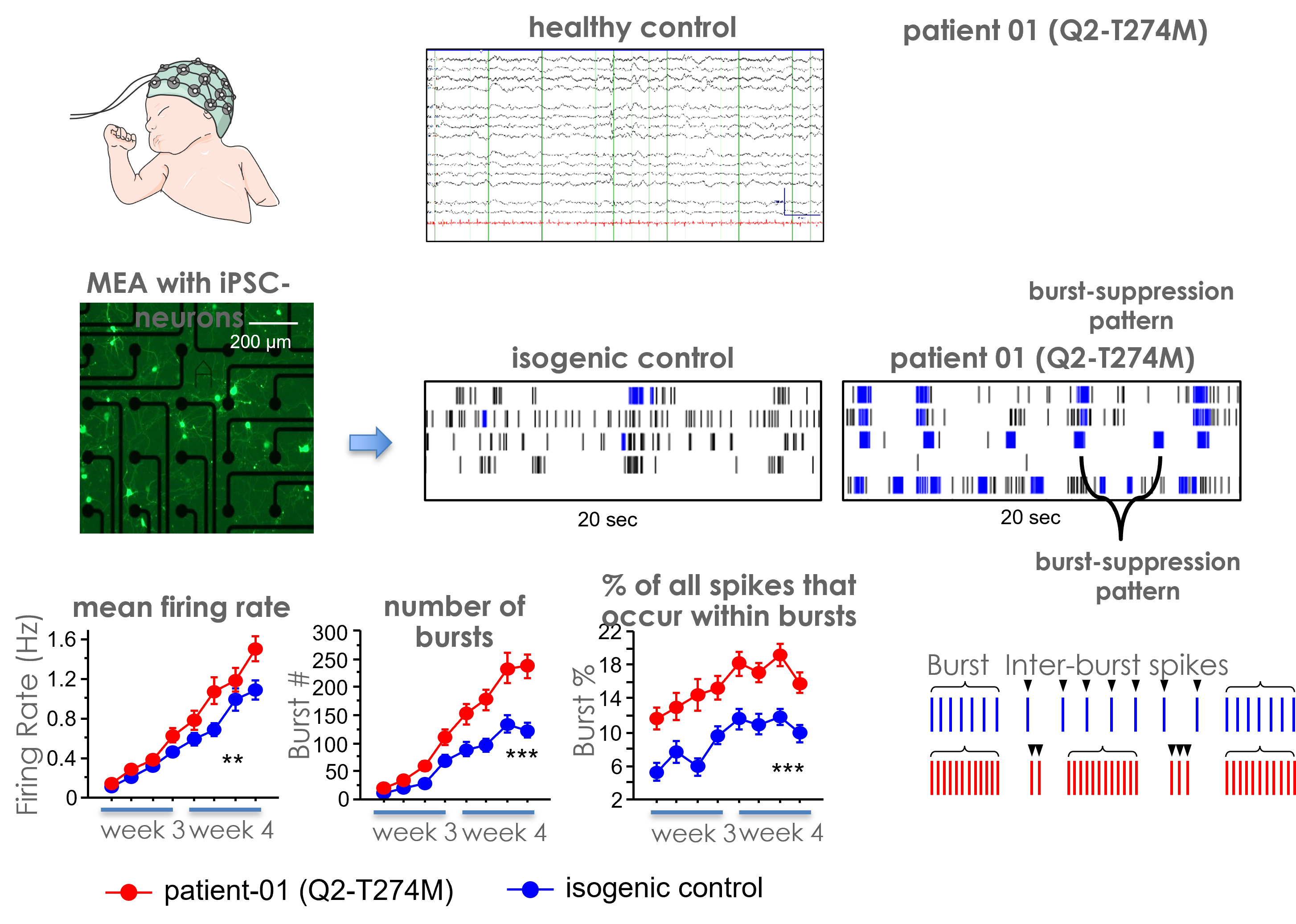
Patient-specific, iPSC-derived neurons, harboring KCNQ2 mutation that results in the KCNQ2-associated epilepsy, were studied on the Maestro MEA platform to assess their firing patterns.
Result: In vitro iPSC-based model exhibited a typical burst-suppression activity pattern, highly reminiscent to the one seen in the brain EEG of the same patient, suggesting its suitability for further phenotypic studies. [Kiskinis, 2019]
Purpose: To investigate the role of isocitrate dehydrogenase (IDH) mutation, which is common among epileptogenic gliomas. Glioma-related seizures occur in more than 70% of patients and may result in a severe deterioration of cognitive function.
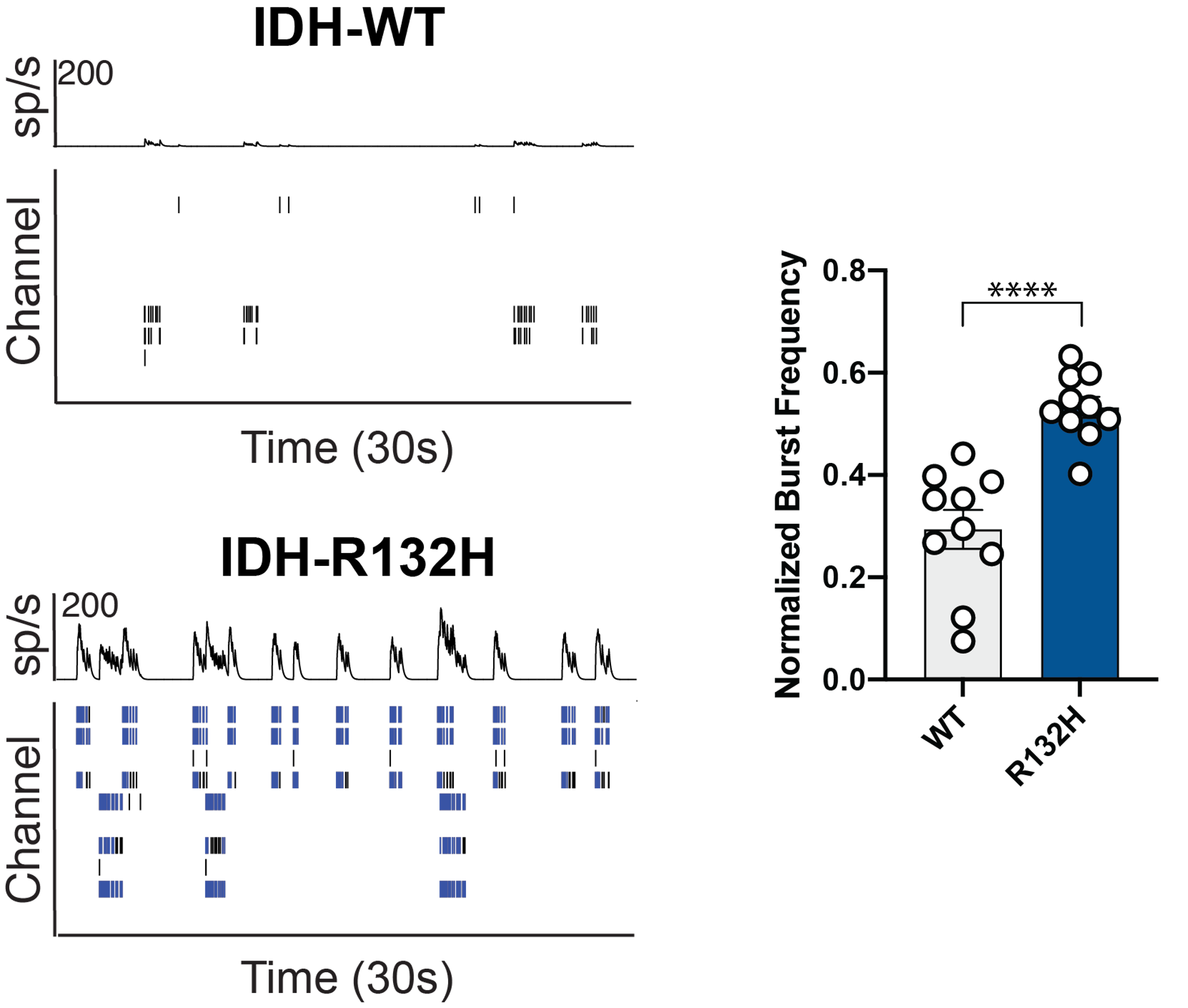
Primary rat cortical neurons were co-cultured with glioma cells with or without an IDH mutation and analyzed using the Axion’s MEA platform.
Result: Maestro MEA data demonstrated that in the presence of glioma cells carrying the IDH-R132H mutation, neurons demonstrated an increase in neuronal bursting activity [Mortazavi 2022].
Purpose: To evaluate seizurogenic potential of compounds. Numerous drugs are associated with adverse side effects, such as seizures and convulsions, advanced, functional assays are needed to predict seizure liability of drugs in vitro.
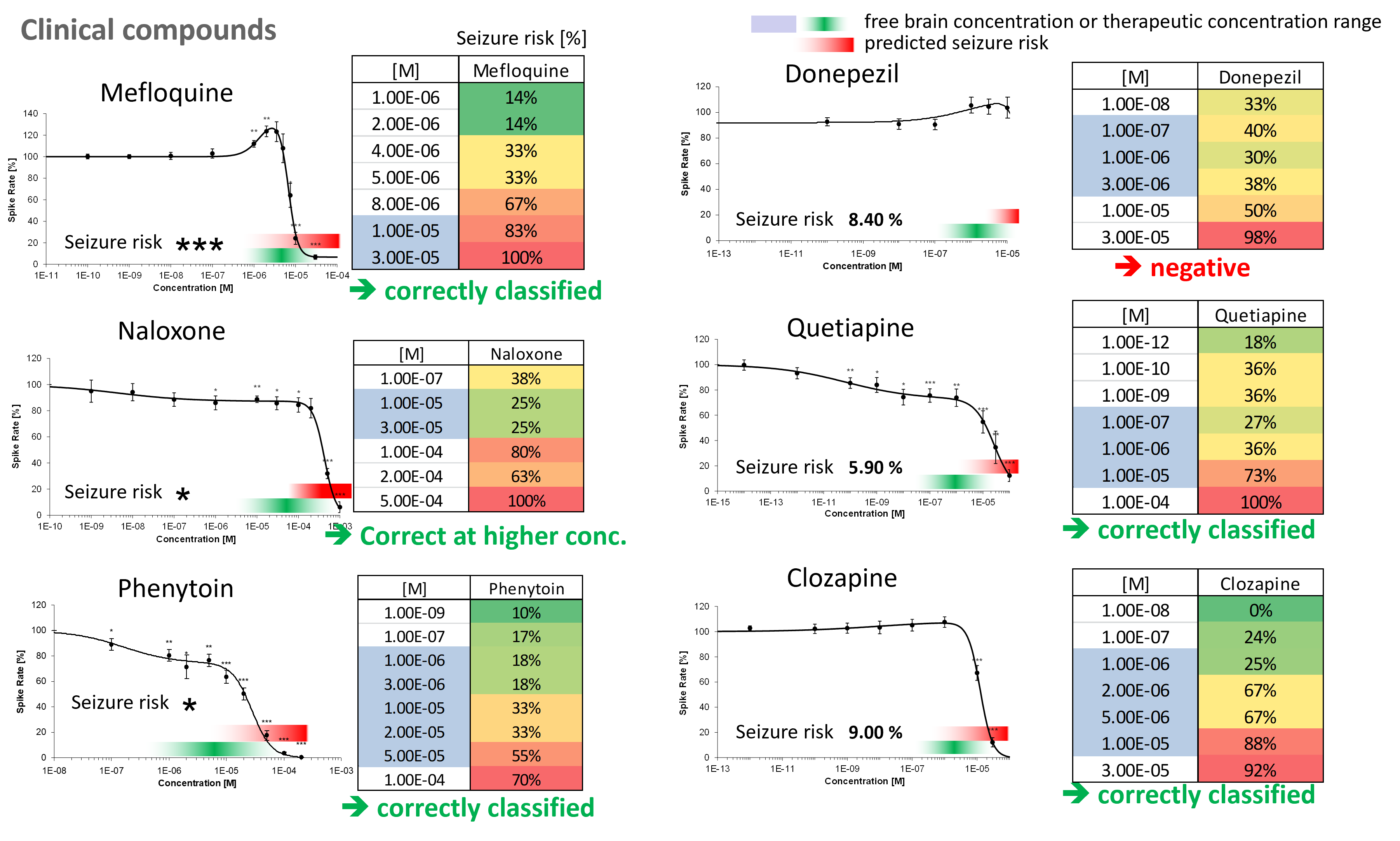
Mouse embryonic brain cells were treated with selected clinical compounds and analyzed via seizure viability prediction assay performed on the Maestro MEA platform. MEA data was analyzed using an artificial intelligence-based protocol.
Result: Compounds were correctly classified using the MEA-based seizure prediction assay. In vitro prediction model also demonstrated correlation with the results of an early clinical trial. [Bader, 2019]

Publication Highlights: Epilepsy
Review the latest epilepsy research using Axion’s platforms and see the impact of real-time functional data. Axion’s Maestro multielectrode array (MEA) platform is enabling scientists to characterize epilepsy and other neurological diseases, both to gain a better understanding of pathogenic mechanisms as well as insight into efficacy of potential therapeutics.
FAQ:
- The Maestro MEA platform offers a controlled environment for studying detailed neural network activity in vitro.
- High-throughput multiwell plates make it ideal for screening patient-specific lines and therapeutics.
- Noninvasive monitoring allows for the study of long-term effects and disease progression.
- It is easy to use, requiring only basic cell culturing techniques to measure neural electrophysiology.
What kind of neural cultures can be measured on the Maestro MEA?
The main requirement is that you have electrically active cells. Primary or stem cell-derived neurons can be used. They may be cocultured with our without glial cells. Neurons can be measured from 2D cultures, organoids or other 3D cultures, and slices.
What kind of metrics can you get from neural activity?
MEA measures from multiple areas of a culture over time, allowing you to go beyond just the firing of individual neurons and evaluate dynamic network activity and the development of functional phenotypes. Learn more about what you can do with our Neural Module.




