In the body, cellular barriers line many surfaces to protect the tissue within and control the passage of material in and out. These barriers are primarily composed of endothelial and epithelial cells. Biological events such as inflammation, infection, cancer metastases, leukocyte migration, and many “normal” cues (e.g., GPCR) can alter the permeability of the barrier.
Axion BioSystems' Maestro Z platform offers real-time monitoring of TEER and barrier function in your cells. Continuous data reveals the full time course of barrier disruption for a more complete picture without the time- and cost-intensive process of repeating multiple endpoint assays.
Measuring in vitro barrier models
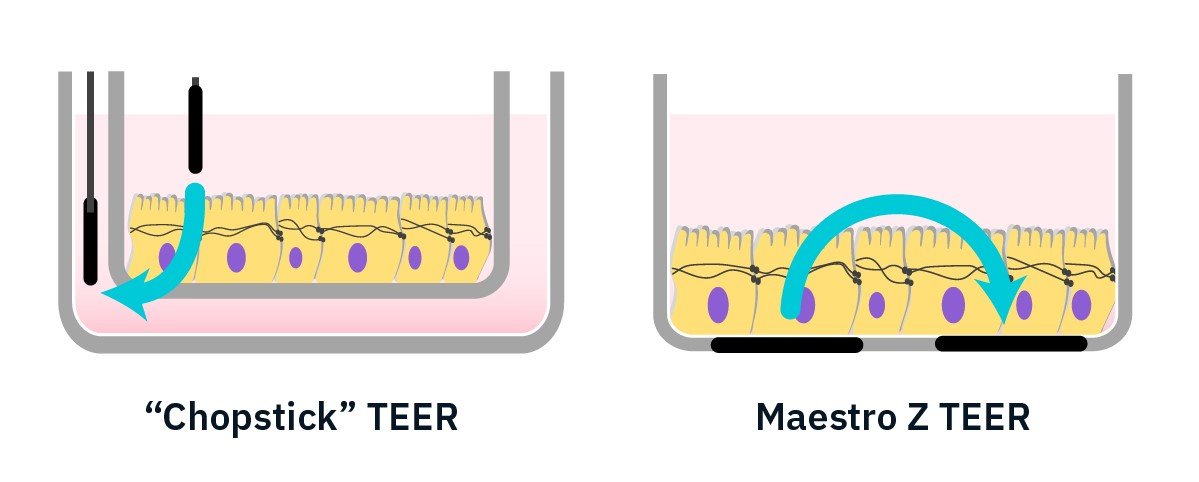
TEER is an important metric to evaluate the integrity of cellular barriers. Handheld “chopstick-electrode” TEER devices can be highly dependent on electrode positioning and manual handling can disturb the cell layer. Integrated interdigitated electrodes on the Maestro Z overcome these challenges.
Advantages of integrated electrodes
- Hands-free
- Reproducible
- High-throughput
Barrier Function Assays
-
TEER Overview>
-
Track barrier integrity in real time>
-
Normalize TEER to cell coverage>
-
Evaluate barrier function in disease-in-a-dish model>
-
Dynamic measurement of microbiome on gut tissue>
Transepithelial electrical resistance (TEER) is commonly used to measure barrier integrity.
The Maestro Z platform can measure TEER and expand on it with barrier index. By simultaneously measuring at different frequencies, the Maestro Z platform calculates barrier index normalizing TEER values for confluence. This provides a sensitive and less variable method to measure small, transient disruptions in barrier function.
Barrier permeability can be altered by many common drugs and signaling molecules.
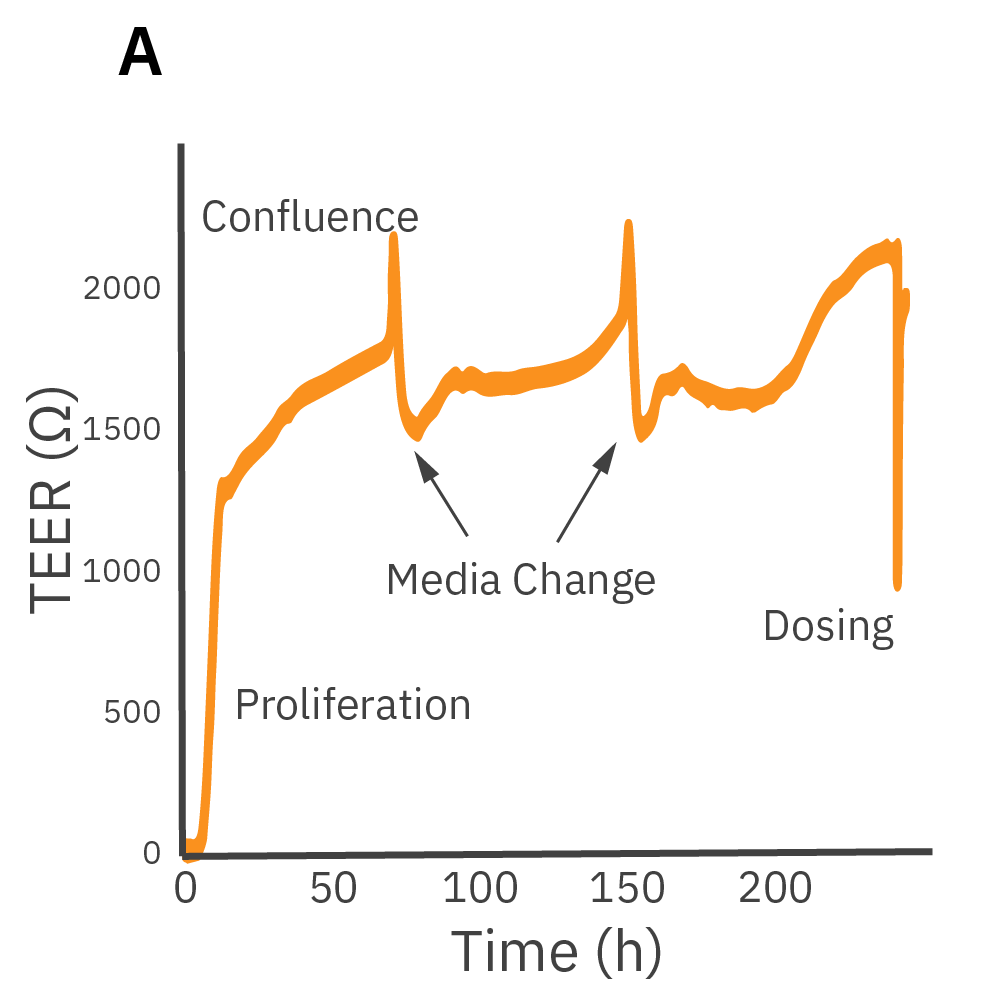
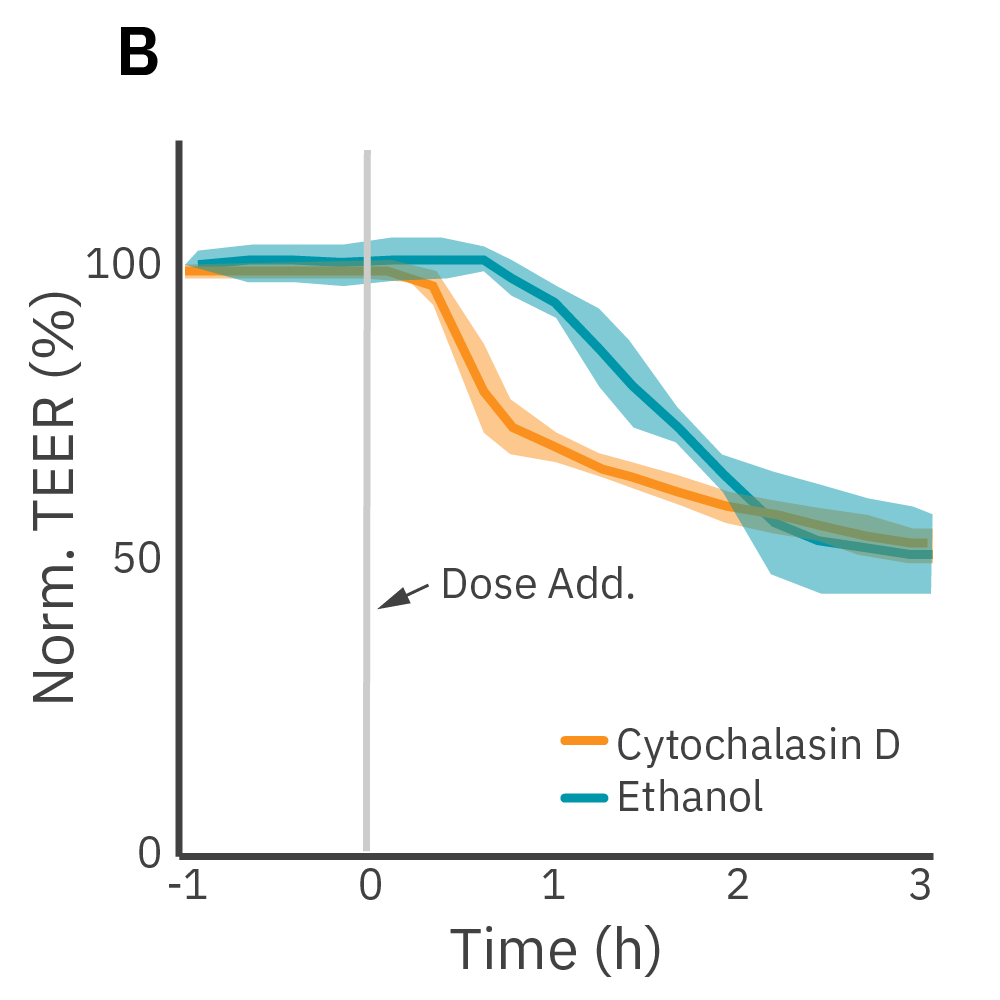
(A) Barrier permeability can be measured continuously from proliferation to confluence. (B) Calu-3 monolayers were cultured for 14 days in the CytoView-Z 96-well plate and then treated with cytochalasin D and ethanol, both of which significantly reduced the TEER.
TEER was monitored continuously on the Maestro Z. After reaching confluence, cells were dosed with cytochalasin D or ethanol.
Results: Cytochalasin D inhibits actin polymerization to increase tight junction permeability. Ethanol acts more slowly on ZO-1 and claudin-1, two key tight junction proteins. Both significantly reduced TEER and barrier disruption demonstrated distinct kinetics between the two compounds.
Purpose: Demonstrate that the Maestro Z can measure coverage and TEER simultaneously. TEER requires confluent cells. By measuring at multiple frequencies, the Maestro Z platform can normalize TEER results to coverage.
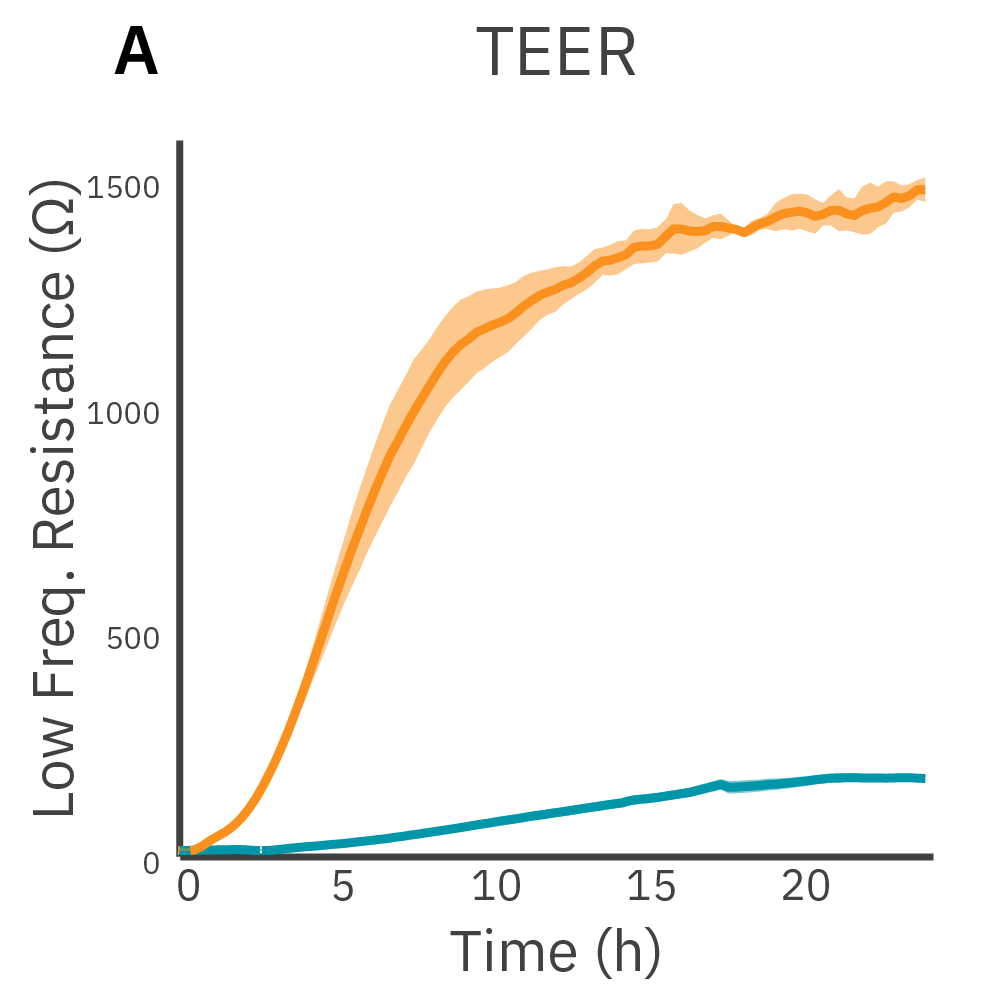
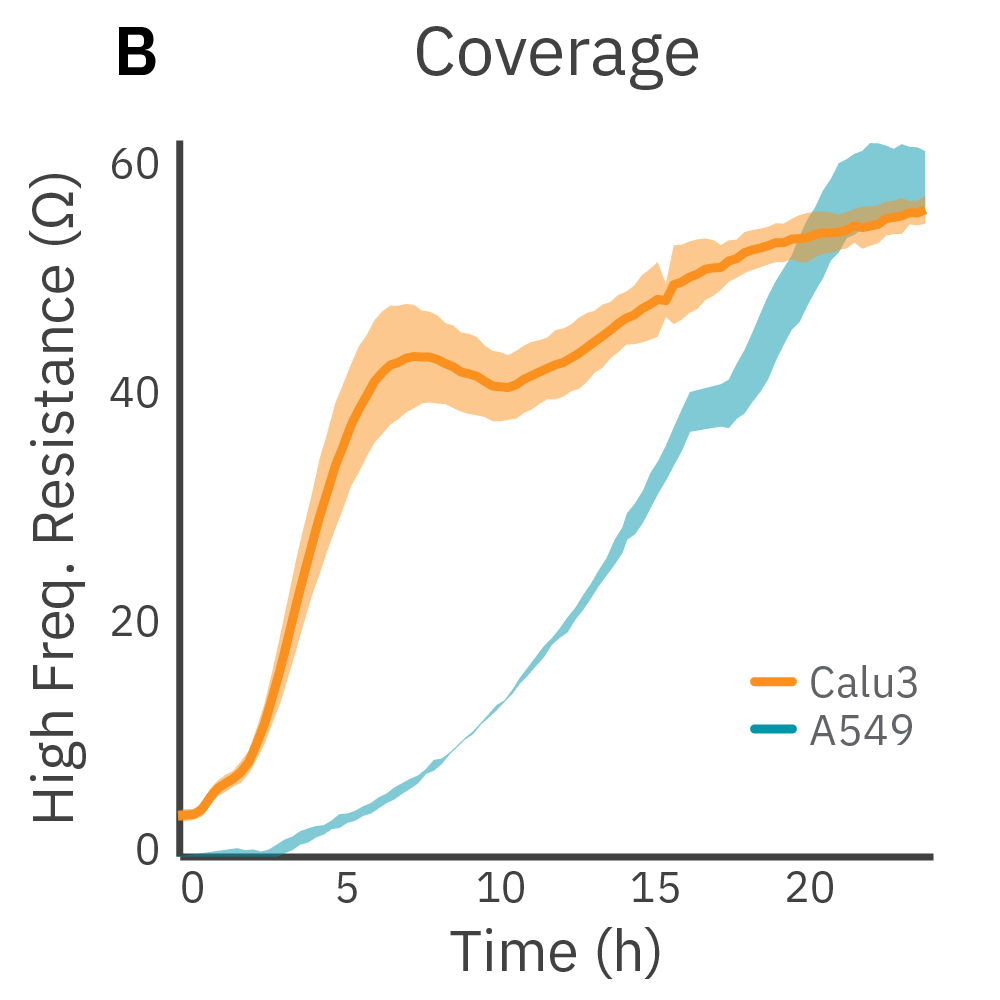
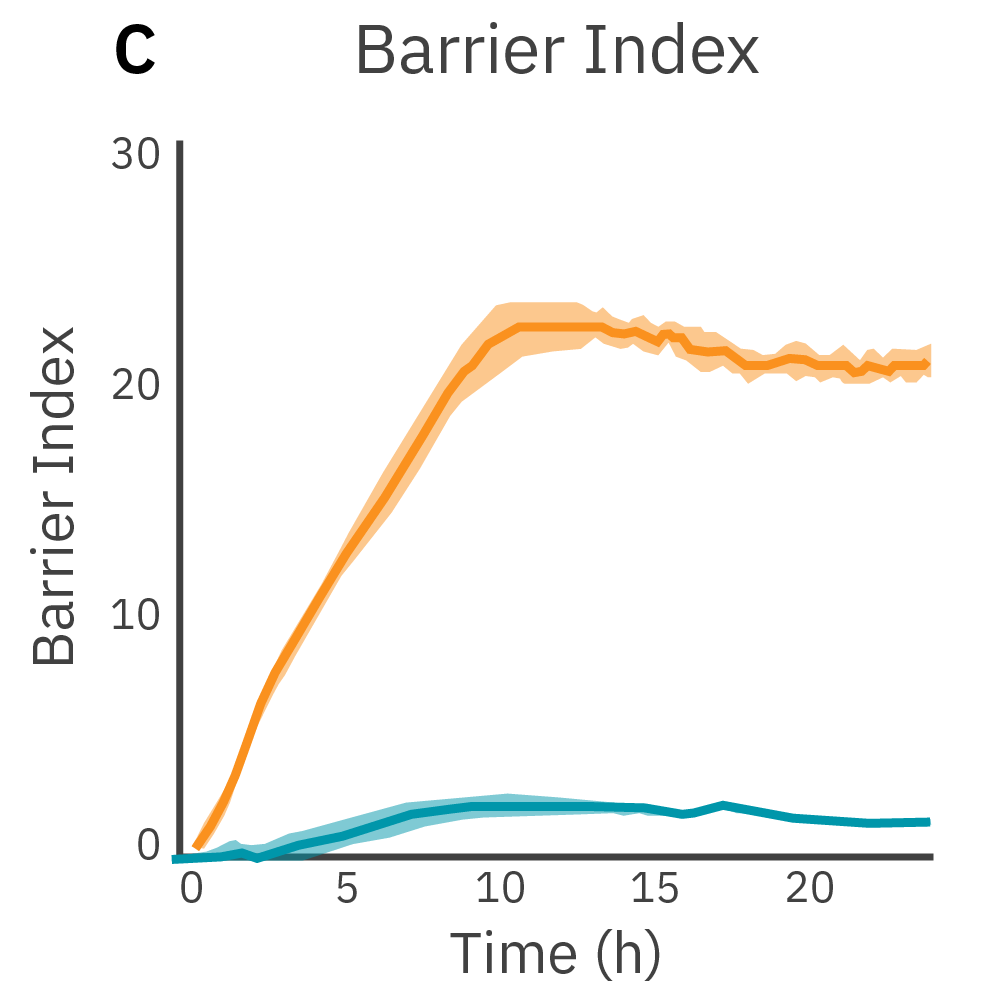
Low frequency measurements are highly sensitive to the intercellular barrier formed by tight junctions and the paracellular barrier formed by cell membranes. High frequency measurements can quantify coverage. By calculating the ratio of resistance at these two frequencies, TEER can be normalized to the cell coverage which is given as barrier index.
Results: Maestro Z continuously and simultaneously monitored cell coverage and barrier function of two human lung epithelial cell lines. Both cell lines reach full coverage within 24 hours. However, only the Calu-3 cells, which express tight junctions, produce a significant low-frequency TEER signal, indicating a strong cellular barrier.
Barrier properties serve critical functions throughout the human body and may be disrupted by diseases, such as cystic fibrosis. Calu-3 is an immortalized cell line that produces tight junctions and expresses functional cystic fibrosis transmembrane conductance regulator (CFTR), which allows ion transport across the cell membrane upon β-adrenergic stimulation [Shen et al., 1994]. TEER measurements capture the function of CFTR, which opens conducting ion channels to facilitate mucus secretion, a process disrupted in cystic fibrosis.
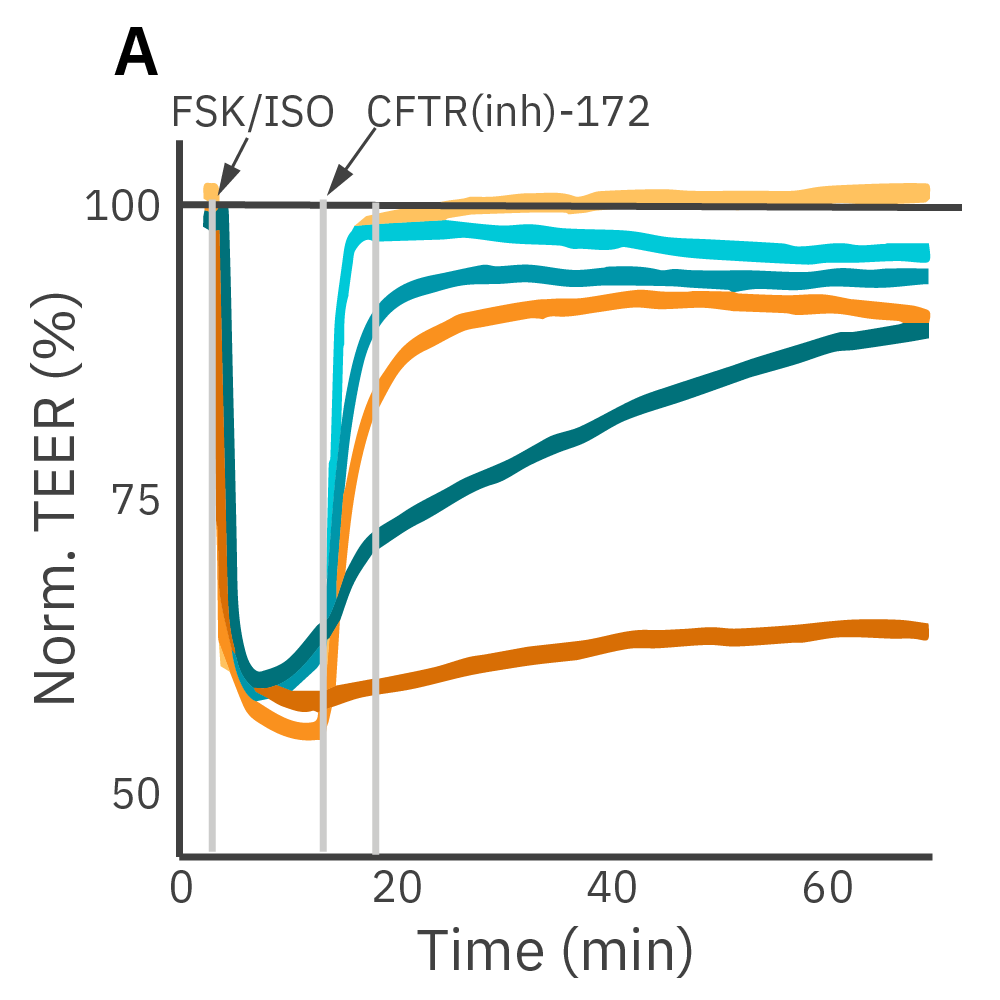
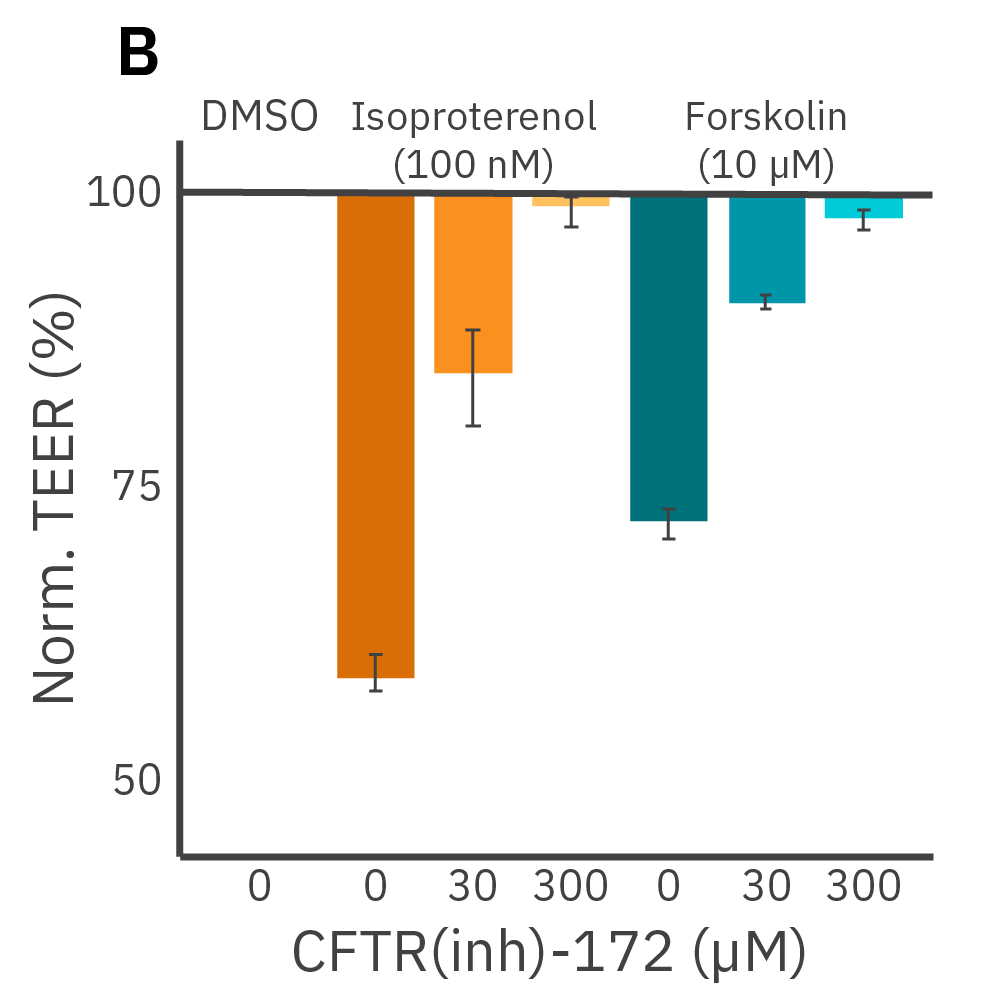
(A) Addition of isoproterenol (teal, 100 nM) and forskolin (orange, 10 µM) significantly reduced TEER of the Calu-3 cells. CFTR(inh)-172 was then added to the plate at 30 and 300 µM causing a rapid dose-dependent reversal of the isoproterenol and forskolin response. (B) 5 minutes after CFTR(inh)-172 addition isoproterenol and forskolin responses are inhibited in a dose-dependent manner.
Purpose: Evaluate the impact of gut microbial taxa on host tissue. Diet alters the composition of the gut microbiome and leads to a clinical response in irritable bowel syndrome (IBS) [Bootz-Maoz, et al., 2022].
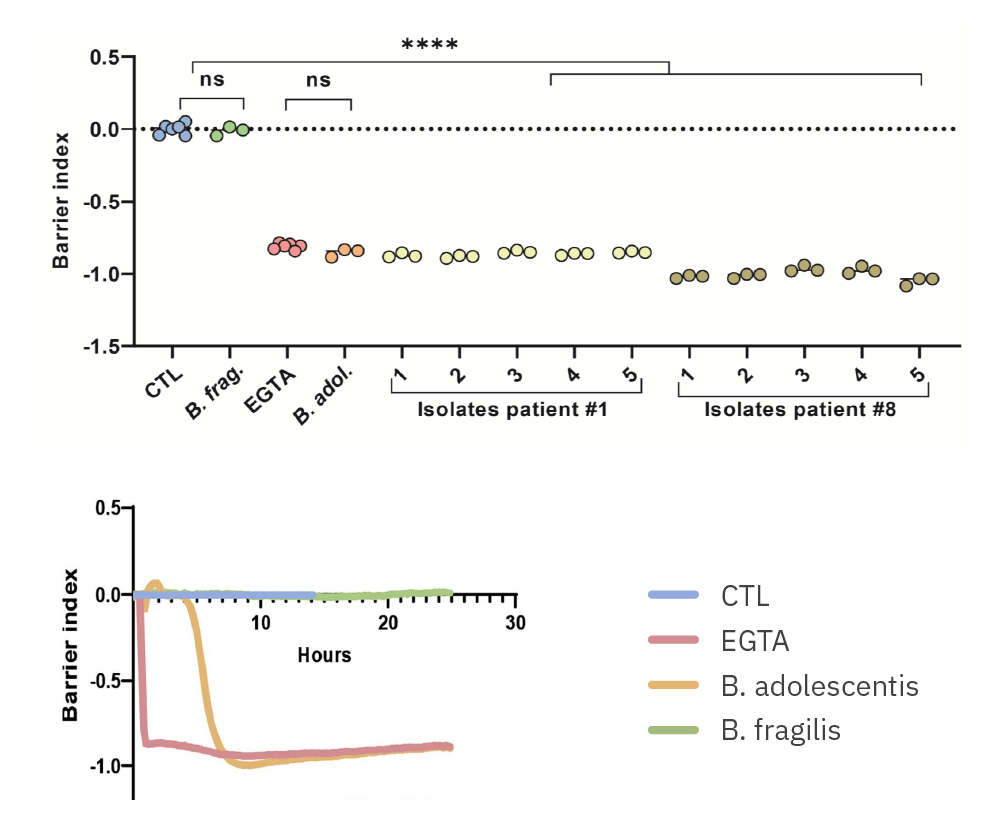
(Images from H Bootz-Maoz, A. Pearl et al, Cell Reports, 2022, DOI: https://doi.org/10.1016/j.celrep.2022.111657 )
(Top) Barrier Index of 10 isolations of B. adolescentis from two patients at 24 hours and (Bottom) a timecourse comparison of B. adolescentis and B. fragilis on intestinal cells.
Results: B. adolescentis lowered the barrier function of intestinal cell cultures with strain dependent effects. B. fragilis did not.
“The Teer assay is a game-changer in cell barrier research.”
The Teer assay is a vital tool for unraveling the mysteries of cellular barriers and advancing our understanding of health and disease in the easiest way possible. We utilize the Maestro Edge system with the Impedance Module to measure TEER in colonic epithelial cells cultured with various bacteria in vitro. Recently, we demonstrated that B. adolescentis bacteria impair epithelial barrier integrity in patients with irritable bowel syndrome (IBS).
- Hadar Bootz-Maoz. Bar Ilan University, Ramat Gan, Israel