Chimeric antigen receptor (CAR) T cell therapy involves the introduction of genetically modified T cells to a patient with the purpose of identifying and targeting cancer cells. Development of successful CAR T cell therapies requires:
- >> Appropriate antigen targeting
>> CAR optimization
>> Predictive potency assessment
>> Immune cell exhaustion/persistence evaluation - To address these limitations and develop a safe and robust immune cell product, our live-cell analysis platforms can be used to measure phenotype, potency, and persistence of CAR T cells.
T Cells and CAR T Cells
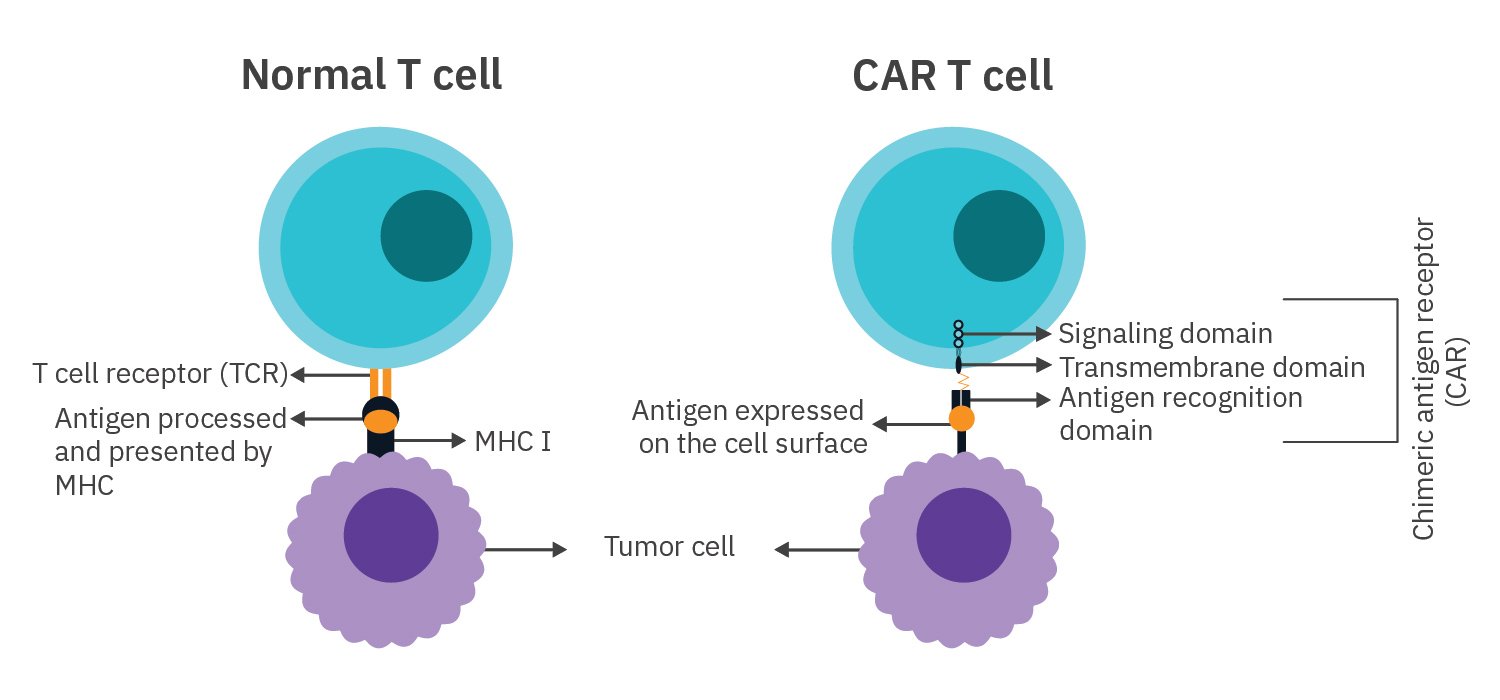
T cell receptors (TCRs) in unmodified T cells detect antigens presented by MHC while chimeric antigen receptors (CARs) in CAR T cells recognize antigens expressed by the target cell.
Structure of a CAR
- Signaling domain — stimulates the activation and proliferation of the T cell
- Transmembrane domain — anchors the CAR to the membrane
- Antigen recognition domain — recognizes and binds to the target cell antigen
CAR T Immunotherapy Assays
-
CAR Optimization with Imaging>
-
Assess specificity of a CAR T cell-mediated response >
-
Measure efficacy against solid tumor spheroids >
-
Evaluate CAR T cell exhaustion>
-
Measure CAR T cell-mediated cytotoxicity>
-
Application Note: Development of a functional cardiotoxicity assay for evaluating the safety of cell therapies>
CAR optimization is critical for effective in vitro immuno-oncology assay development. The Omni live-cell imaging system was used to compare the killing of different CAR constructs and showed that cell death was observed in a dose-dependent manner.
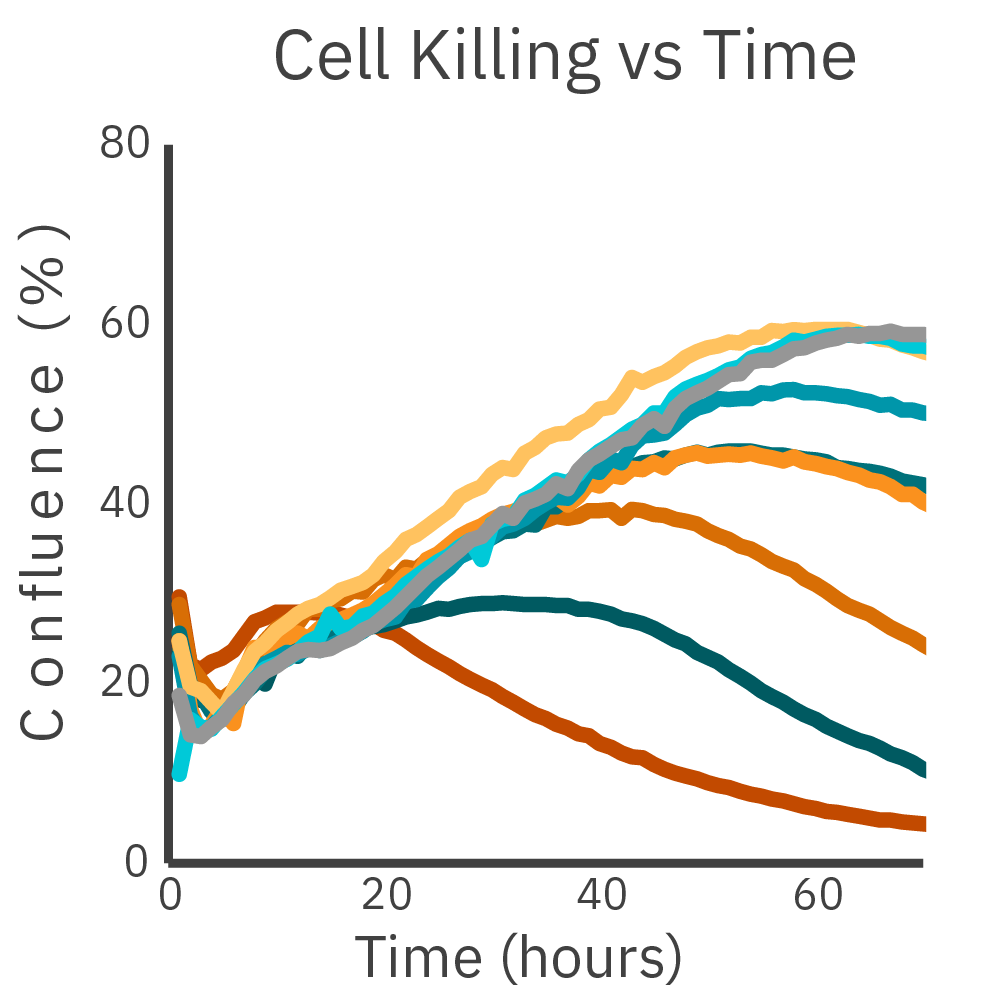
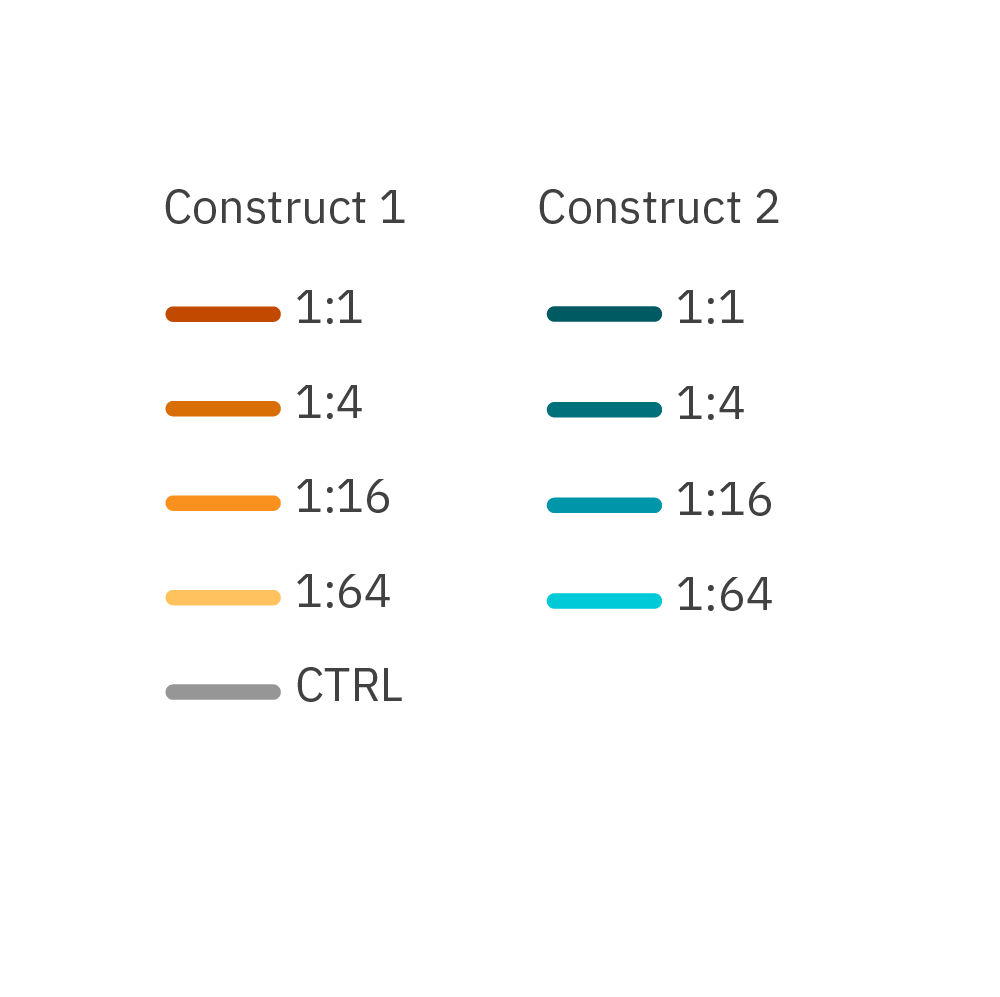
Confluency of RFP-labeled cancer cells after the addition of CAR T cells is measured with the Omni platform. Two different CAR constructs were compared across E:T ratios and Construct 1 showed a greater potency across all ratios.
Purpose: To differentiate between non-specific and specific killing of target cells by CAR T cells. High specificity of CAR T cell-mediated killing ensures safety and effectiveness of a CAR T cell therapy.
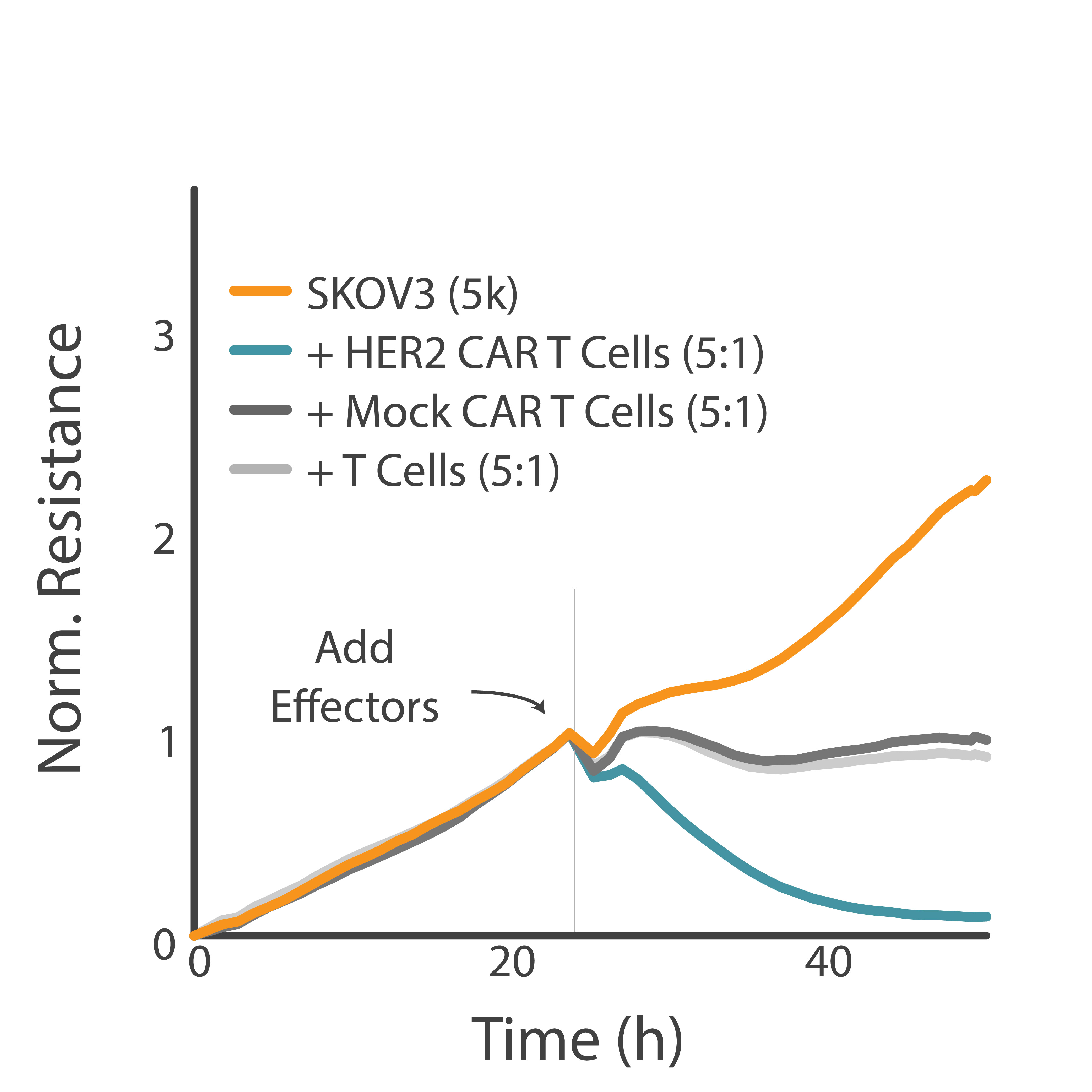
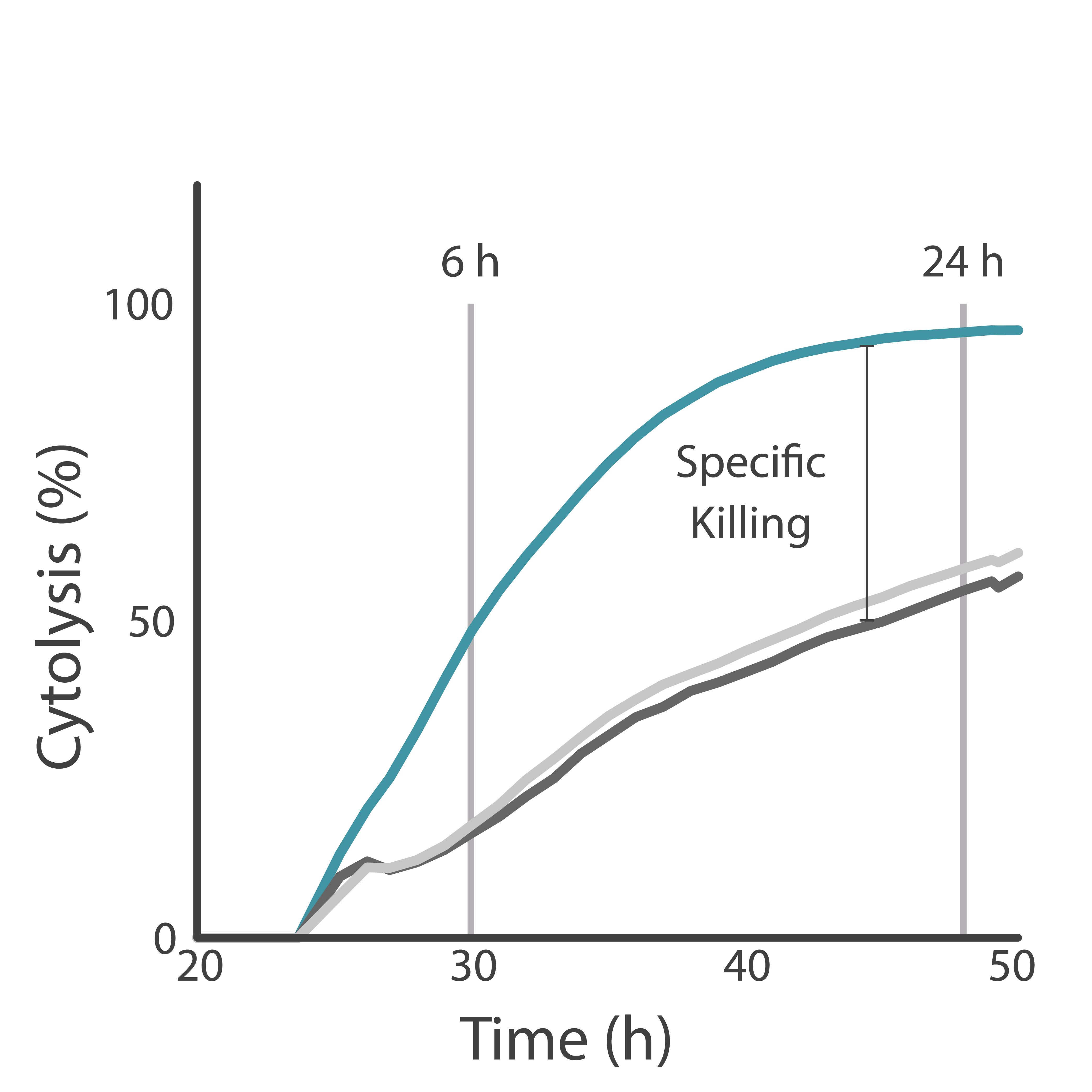
HER2-overexpressing cancer cells were treated with CAR T cells with or without the tumor antigen-recognizing domain. CAR T cell-mediated killing of cancer cells was analyzed using the Maestro Z platform.
Result: CAR T cells possessing the antigen-recognizing domain demonstrated targeted killing of HER2-cancer cells.
Purpose: To track CAR T cell killing in 3D spheroids. Solid tumors can pose additional challenges to immunotherapies compared to liquid tumors or 2D monolayer cultures.
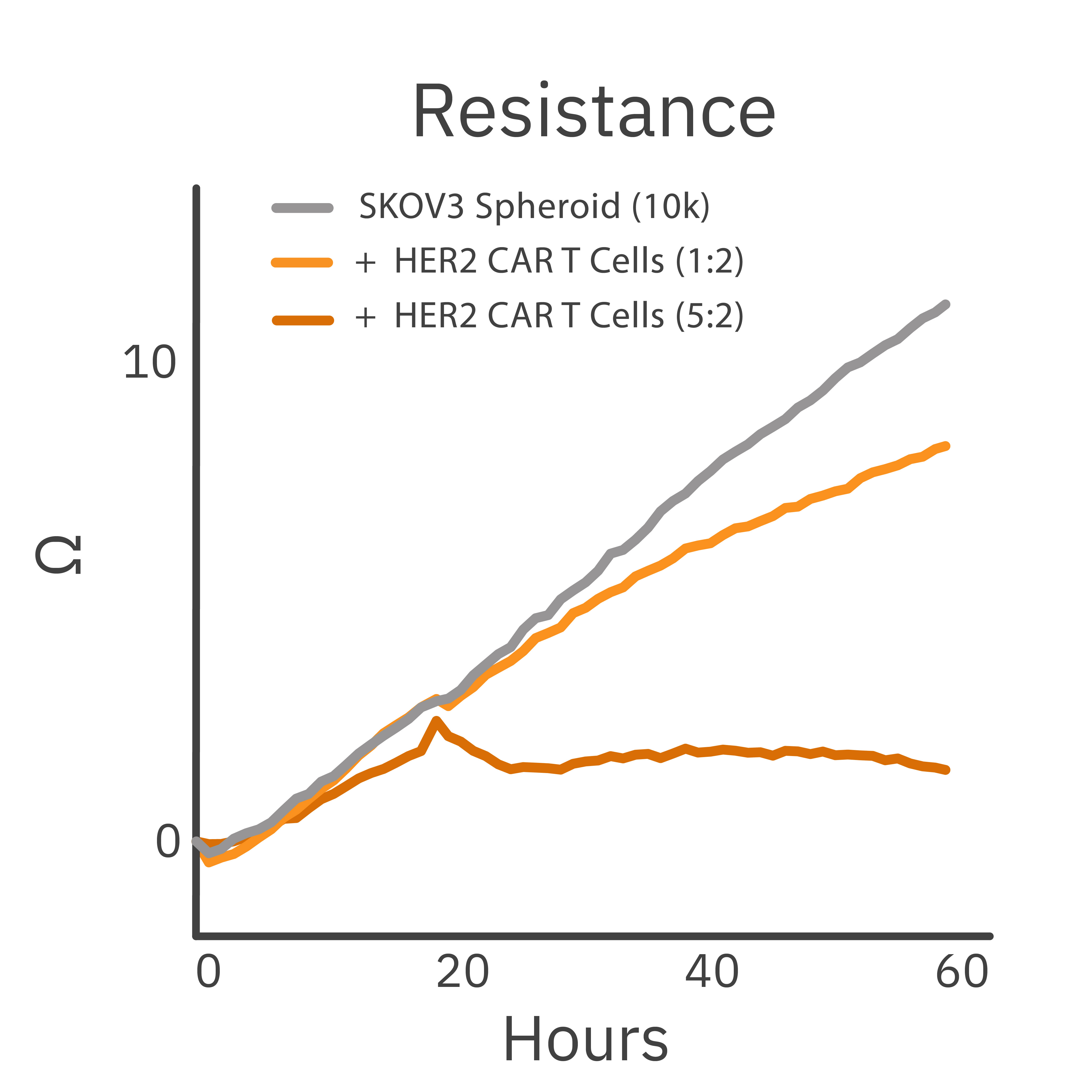
A cancer spheroid was treated with CAR T cells. Cancer spheroid cytolysis was measured using the Maestro Z platform.
Result: A dose-dependent decrease in cancer spheroid size was observed following CAR T cell treatment.
Purpose: To evaluate the persistence of CAR T cell killing. T cell exhaustion is a common hurdle that impedes complete tumor eradication in a patient.
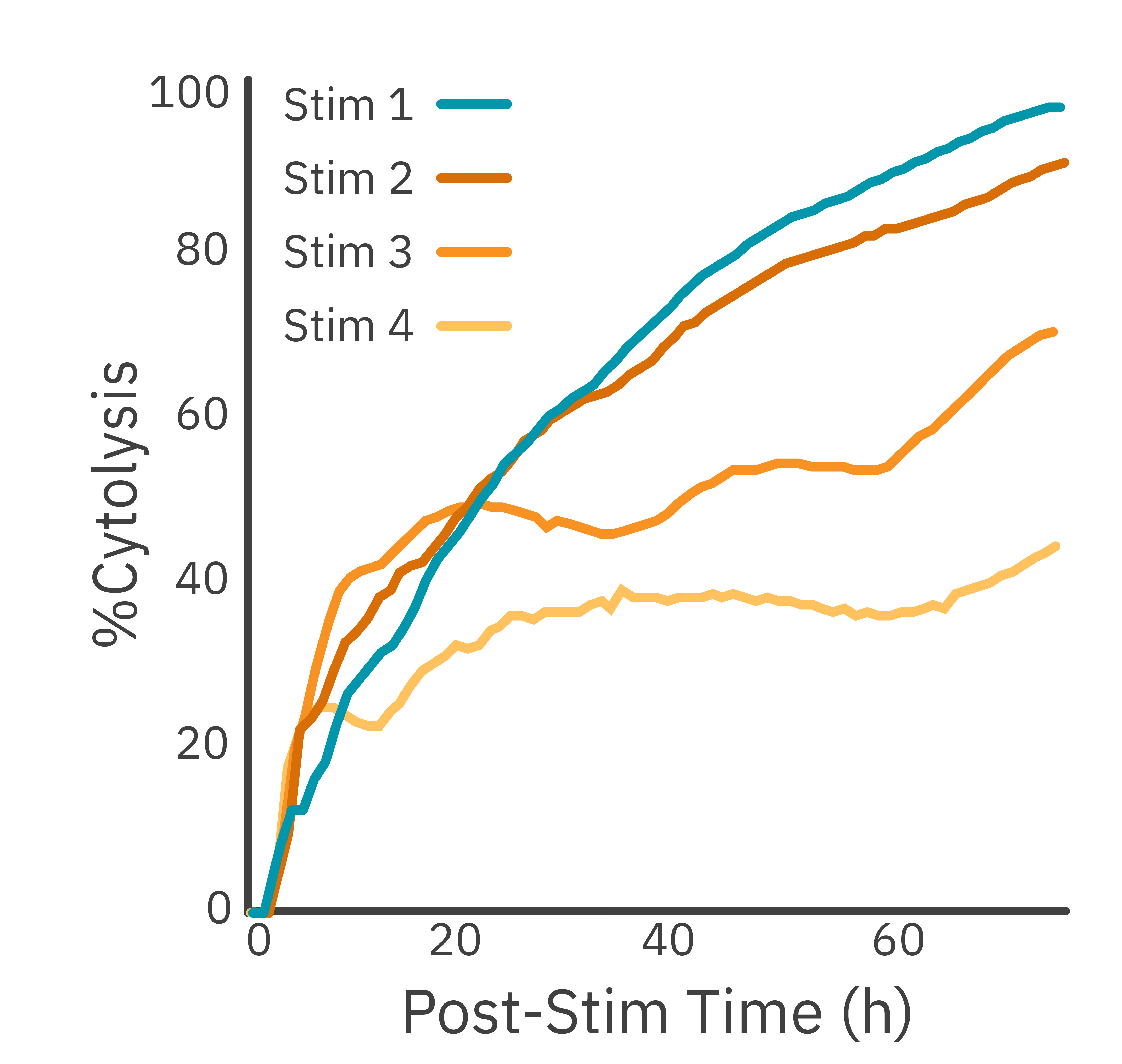
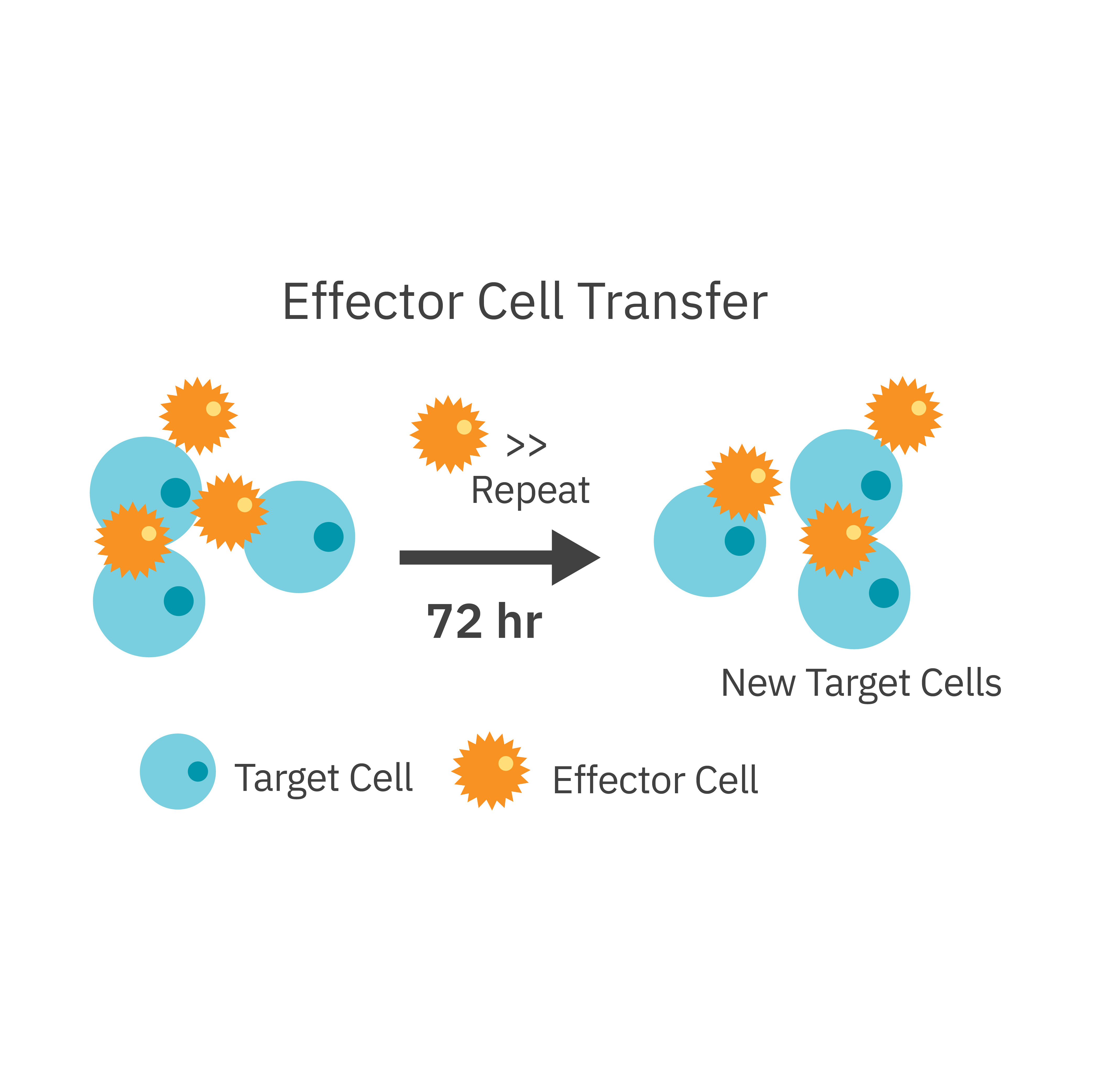
Effector CAR T cells were added to target cancer cells. Every 72 hours they were transferred, at the same ratio, to new target cells. Target cell killing was assessed using the Maestro Z platform.
Result: After each sequential transfer, cytolysis decreased as the CAR T cells entered a less potent state.
Purpose: To measure the potency of CAR T cell-mediated killing of cancer cells expressing different levels of HER2 antigen. Antigen receptor levels have been shown to influence CAR T cytolytic activity.
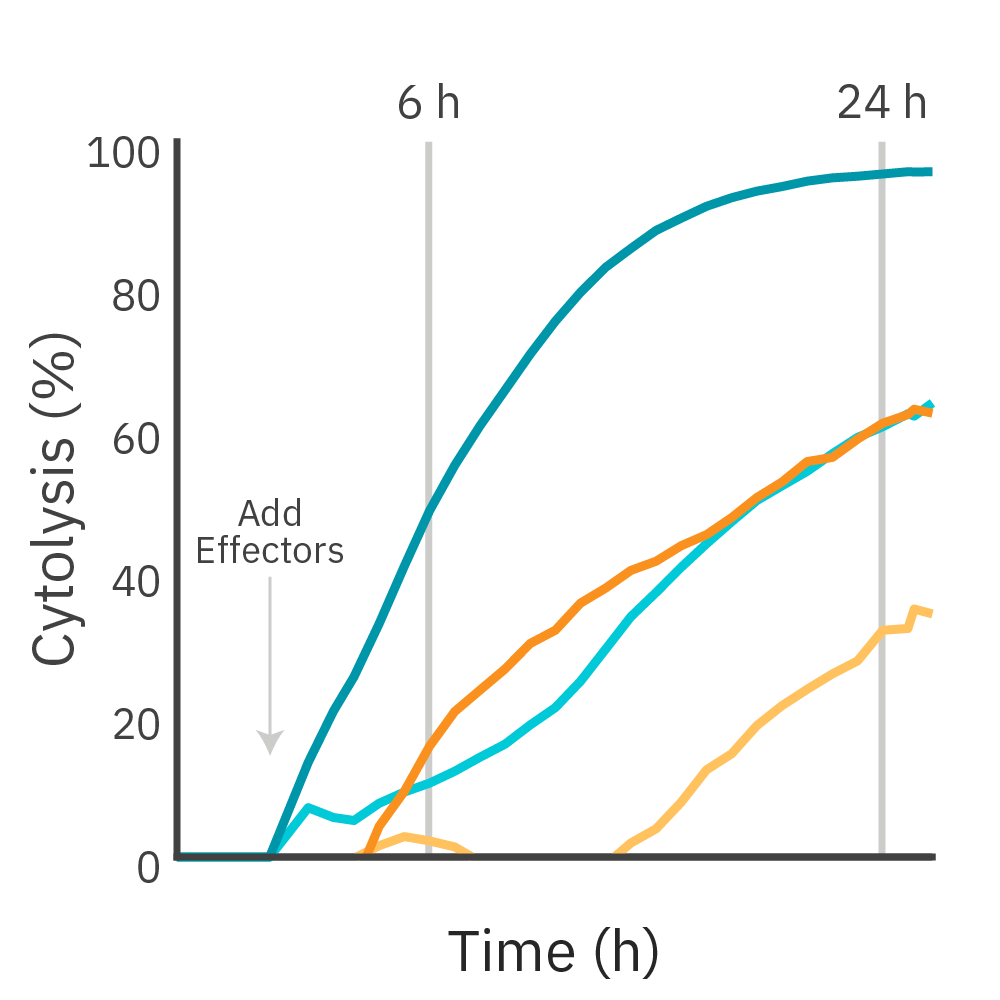
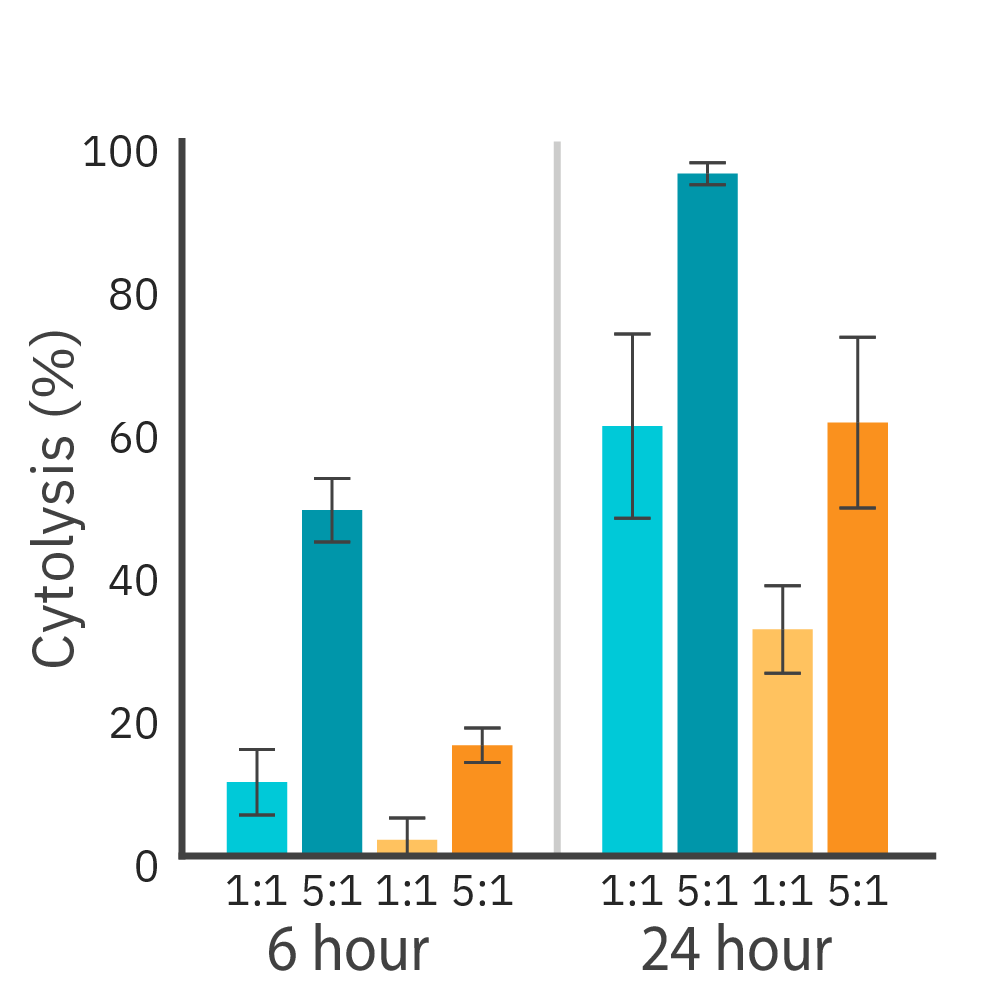

CAR T cells targeting HER2 antigen were added to two cancer cell lines and the magnitude of cell killing was compared using the Maestro Z platform.
Result: CAR T cell-mediated killing was observed against both target cell lines and across different effector-to-target ratios. The rates of cytolysis were directly proportional to the levels of target antigen expression.

In this app note you will learn how:
>> HER2-targeted CAR T cells kill iPSC-derived cardiomyocytes in vitro
>> Non-transduced T cells show no toxicity toward iPSC-CMs, and HER2-CAR T cells do not kill iPSC-derived neurons
>> HER2-CAR T induce electrophysiological changes in iPSC-CMs, often before changes in viability are observed

“Great results!”
Quantitative data with a convenient mobile app. The Maestro Z provides a quantitative measurement of CAR-T cell-mediated cytotoxicity. The system is very easy to use, and it's powerful to see CAR-T cell killing profiles over time. The number of experiments you can run continuously with majority hands-off time is incredible. The mobile app is also great for making decisions about when to come into the lab when to add effectors, and so on.
- Heather Lin. Emory University, Georgia, USA