Dorsal root ganglion (DRG) cultures exhibit chemical and thermal sensitivities representative of those observed in vivo and can be cultured in vitro in Axion’s multiwell microelectrode array (MEA) plates, enabling simultaneous recording of electrical activity from numerous test conditions. In combination, DRG neurons and the Maestro multiwell MEA system constitute a high-throughput in vitro platform for chronic neuropathic pain research.
The sensation of pain is transmitted from sensory nerve endings to the central nervous system by axons of peripheral neurons whose cell bodies reside in the dorsal root ganglion (DRG). Damage to these primary afferent neurons, or defects in the proteins underlying their electrical or sensing function, can cause neuropathic pain, a persistent sensation of pain associated with increased spontaneous firing of DRG neurons
-
MEA recordings capture the transient and persistent increases in firing rate of DRG neurons induced by capsaicin>
-
Publication Highlights: Pain Research>
-
TRPV1 antagonists modulate the effect of capsaicin on DRG neuron activity>
-
Thermal sensitivity of DRG neurons recorded on an MEA system>
-
Burning Man Syndrome neurological activity increases as temperature increases>
-
Assay Steps>
Sensitivity of DRG neurons to noxious stimuli is largely caused by the TRP family of ionotropic receptors, which display differing sensitivity profiles for stimuli including chemical agents, noxious heat and cold, and changes in pH due to inflammation. Of particular relevance is the TRPV1 channel, a primary receptor for noxious heat and pH implicated in hyperthermia and inflammatory pain. Capsaicin is an active component of chili peppers, and is a chemical irritant for humans, producing a sensation of burning in any tissue with which it comes into contact. Activation of DRG neurons was conducted using capsaicin, an agonist of the TRPV1 receptor. The effect of 100 nM capsaicin on mean firing rate (MFR) of DRG neurons (1x104 cells per well) was measured on DIV 7 with Axion's MEA system.
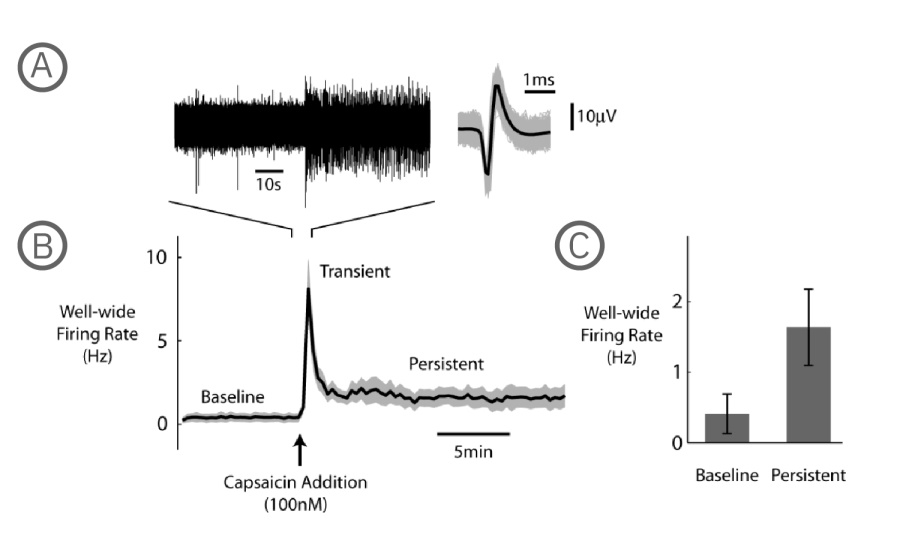
A) The raw voltage trace shows the increased firing resulting from capsaicin addition, with the waveform of each detected spike plotted to the right (gray), along with the mean spike waveform (black). B) Three separate stages of the capsaicin response are apparent in the plot of well-wide firing rate (N=6 wells, mean – black, +/- standard error of the mean – gray): baseline spontaneous firing, transient capsaicin-induced firing, and a persistent elevated firing that lasts for tens of minutes following the capsaicin addition. C) The bar chart represents an average over a 3 minute period for both the baseline and the persistent phases of the capsaicin response, where the firing rate was higher in the persistent phase (N=6, p = 0.0313, Wilcoxon Signed Rank Test, error bars represent standard error of the mean).
In summary, Axion's multiwell MEA system with the Neural Module recorded the significant well-wide increase in DRG firing rate after the addition of capsaicin.

Publication Highlights: Pain Research
The need for personlized treatments for chronic pain is growing. Download highlights from five publications by researchers using the Maestro platform to learn more about stem cell models, personalized treatments, and novel therapeutic targets.
Robust in vitro activation of DRG neurons by capsaicin sets the stage for screening assays in which this response is blocked by potential pain therapeutics. Two wells of DRG neurons (1x104 cells per well) cultured in MEA plates were treated with 100 nM capsaicin at 7 minutes, causing an increase in firing rate. After 20 minutes, DRG neurons were treated with 10 mM dehydroandrosterone (DHEA), a TRPV1 competitive inhibitor. The DHEA treated DRGs exhibited a significant reduction in mean firing rate compared to the control well, consistent with previous whole cell patch clamp studies on DRG neurons. After 10 minutes of reduced firing due to DHEA exposure, the effect was markedly reversed by addition of 1 mM capsaicin, demonstrating continued sensitivity of the TRPV1 receptors.
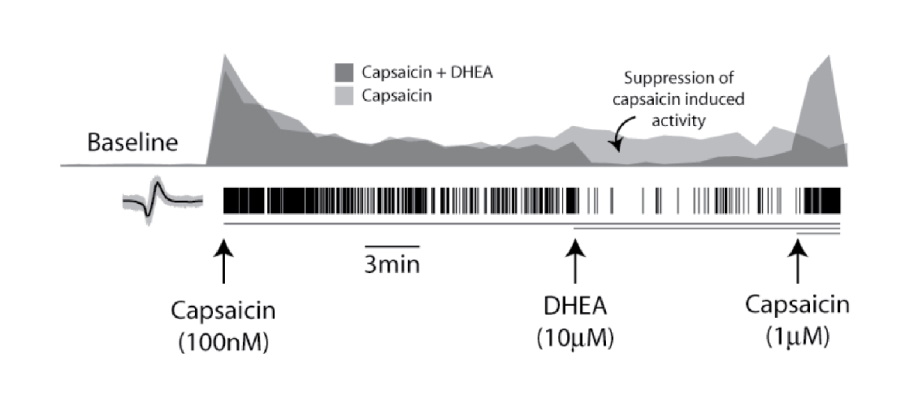
The histograms represent the well-wide firing rate normalized by the capsaicin induced activity (bin size of 60 secs). Addition of a higher dose of capsaicin (1mM) rescued the firing activity suppressed by DHEA (dark gray).
Modulation of DRG neuron activity, suppression and rescue of firing activity, was recorded across a microelectrode array well.
Noxious heat is a modulator of TRPV1 channel conformation. Temperatures in excess of 43°C lower its activation threshold, causing increased excitability of the neuron. The thermal response of DRG neurons can be evaluated on the Maestro MEA system using the integrated temperature control, which directly heats the MEA plates. The responses of 5 individual DRG neurons exposed to increasing temperature were analyzed by first spike sorting in the Plexon Offline Sorter and then loading the sorted spike trains into the Neural Metric Tool.
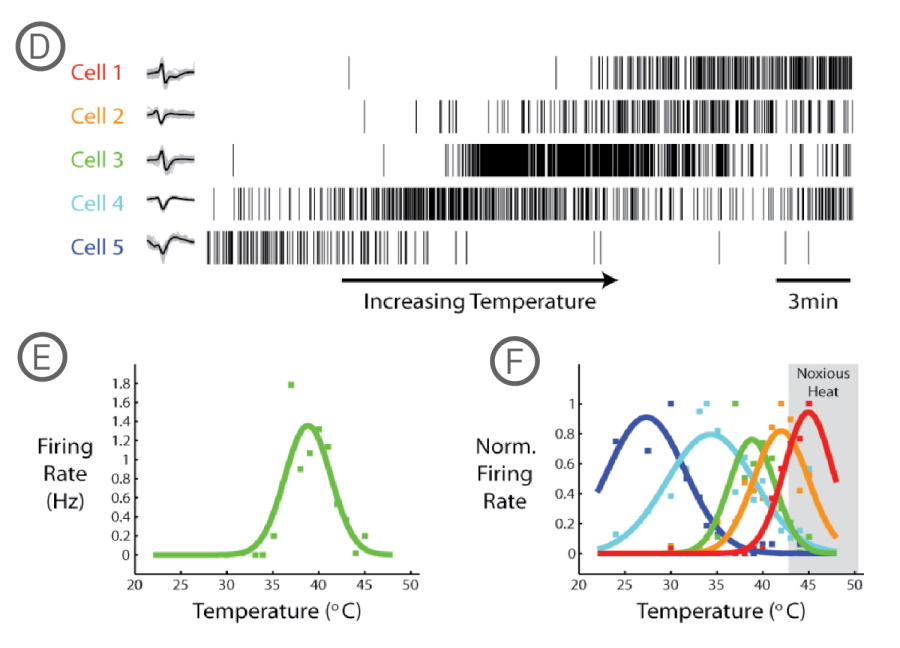
Spike raster plots for 5 different DRG neurons recorded over time as the temperature was ramped from 24°C to 45°C. B) MFR for Cell 3 (green) at different temperatures with a Gaussian curve fit (bin size = 60 secs). C) Normalized firing rate for the 5 cells in A, color-coded with Gaussian curve fits to show temperature sensitivity ranges.
Maestro MEA system's ability to change the plate temperature through integrated temperature control showed correlated temperature sensitivity ranges in the firing of DRG neurons.
Neurons expressing a specific mutation associated with inherited erythromelagia, a chronic pain syndrome characterized by a burning sensation in response to heat, were cultured on the MEA and treated with carbamazepine which reduced their sensitivity to temperature increases. Two patients with the same mutation were then treated with carbamazepine and reported a reduction in pain duration when compared to a placebo [Mis et al. 2018].
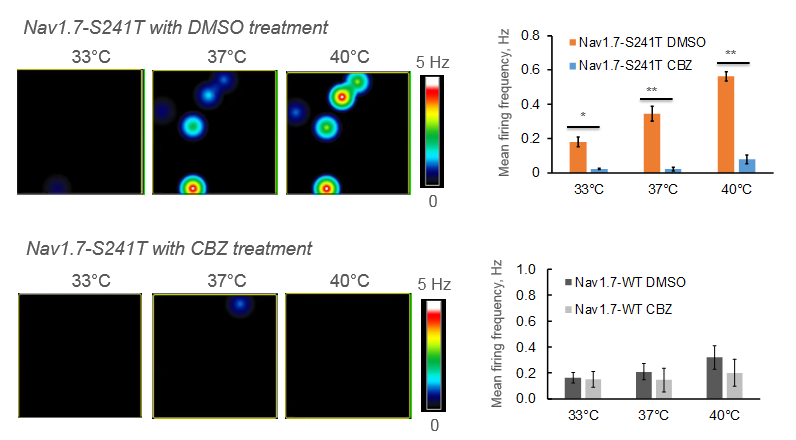
Maestro microelectrode arrays recorded the change in mean firing frequency of the burning man syndrome neurons in response to suppression treatment as the multiwell MEA plate environment increased in temperature.
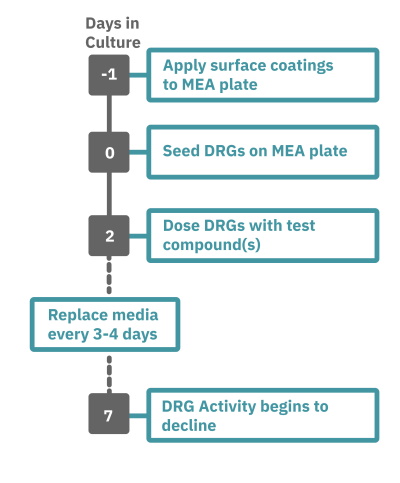
Getting started with Maestro Pro and Edge couldn't be easier. Culture your DRG neurons in an Axion multiwell MEA plate (Day 0). Load the MEA plate into the Maestro MEA system at the desired recording times, allow the built-in environmental controls to equilibrate, and begin recording. Analyze the neural activity in the MEA plate, label-free and in real-time, with AxIS Navigator Neural Module software.






