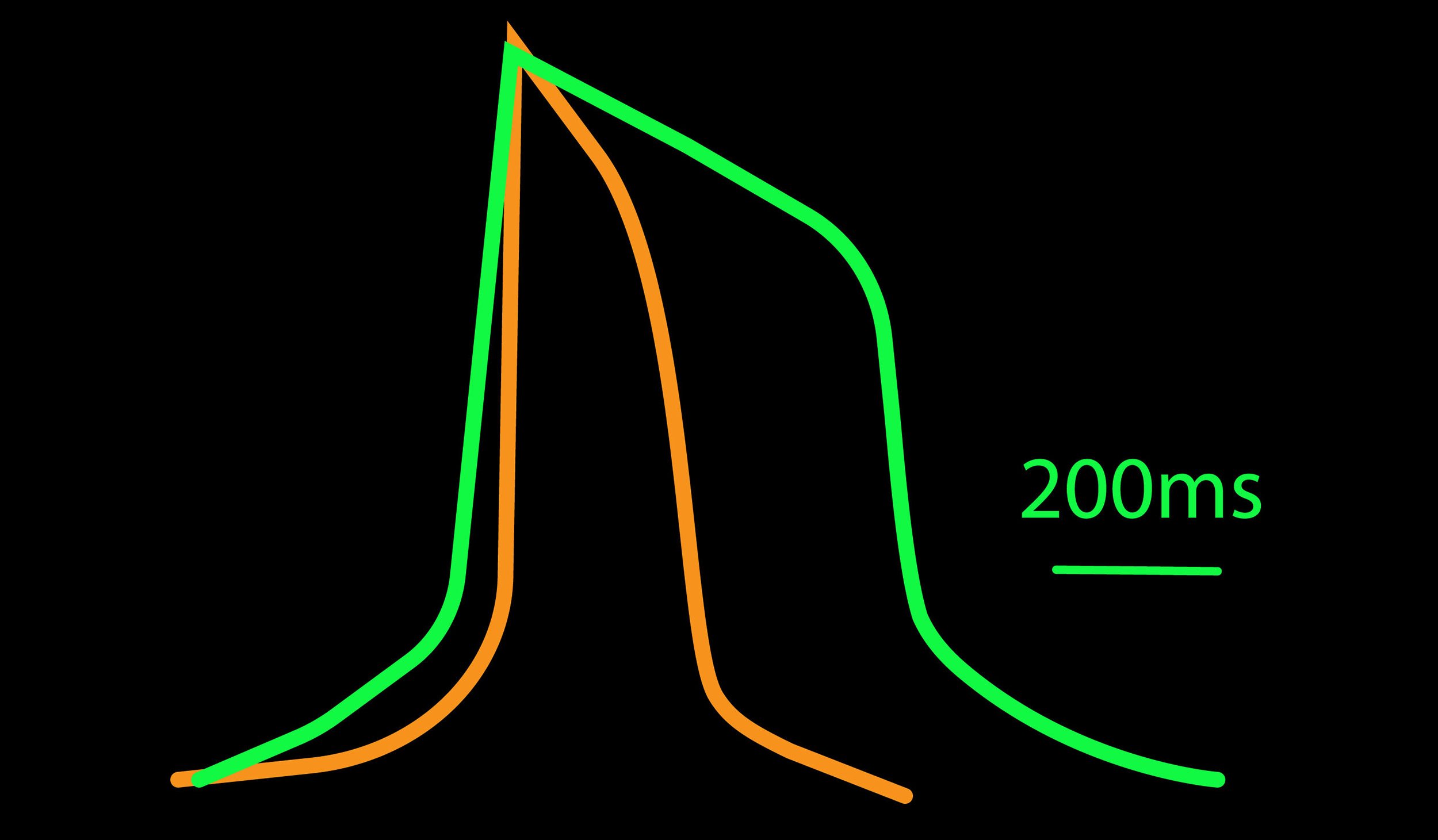
心筋細胞から得られる活動電位は、培養内の心筋細胞のサブタイプを識別するのに有用です。心筋細胞の活動電位は、脱分極、プラトー、再分極のフェーズで構成されます。これらのフェーズの持続時間やカイネティクスの差異により、心房筋様、結節様、心室筋様など心筋細胞の識別が可能です。例えば、心房筋細胞のプラトーフェーズは短く、再分極フェーズの傾斜は鋭く、活動電位の持続時間は短く三角形的(triangular)な形状をしています。一方、心室筋細胞の活動電位は、顕著なプラトーフェーズが特徴で、持続時間も長く、四角的な波形をしています。
心臓における異種の心筋細胞は、その活動電位の差により一定程度の分類が可能です。例えば、典型的に、心房筋様細胞は、心室筋様細胞と比較して、短いプラトーフェーズと持続時間 (APD90) を示します。
Maestro MEA の LEAP (Local extracellular action potential) は、MEA電極を用いてAction Potential 形態シグナルを測定します。パッチクランプで要する電極操作は不要。ラベルフリーで測定し、活動電位波形の解析が行えます。
LEAPによる心筋細胞からの活動電位形態シグナルの測定と解析
心筋細胞の研究及び安全性試験などにおいて、活動電位の測定と解析は極めて重要です。本事例では、4種類のiPS細胞由来心筋細胞及び齧歯動物から得られた心筋細胞から、Maestro を用いて活動電位形態シグナルを測定しました。細胞種毎に特徴的な波形が得られました。
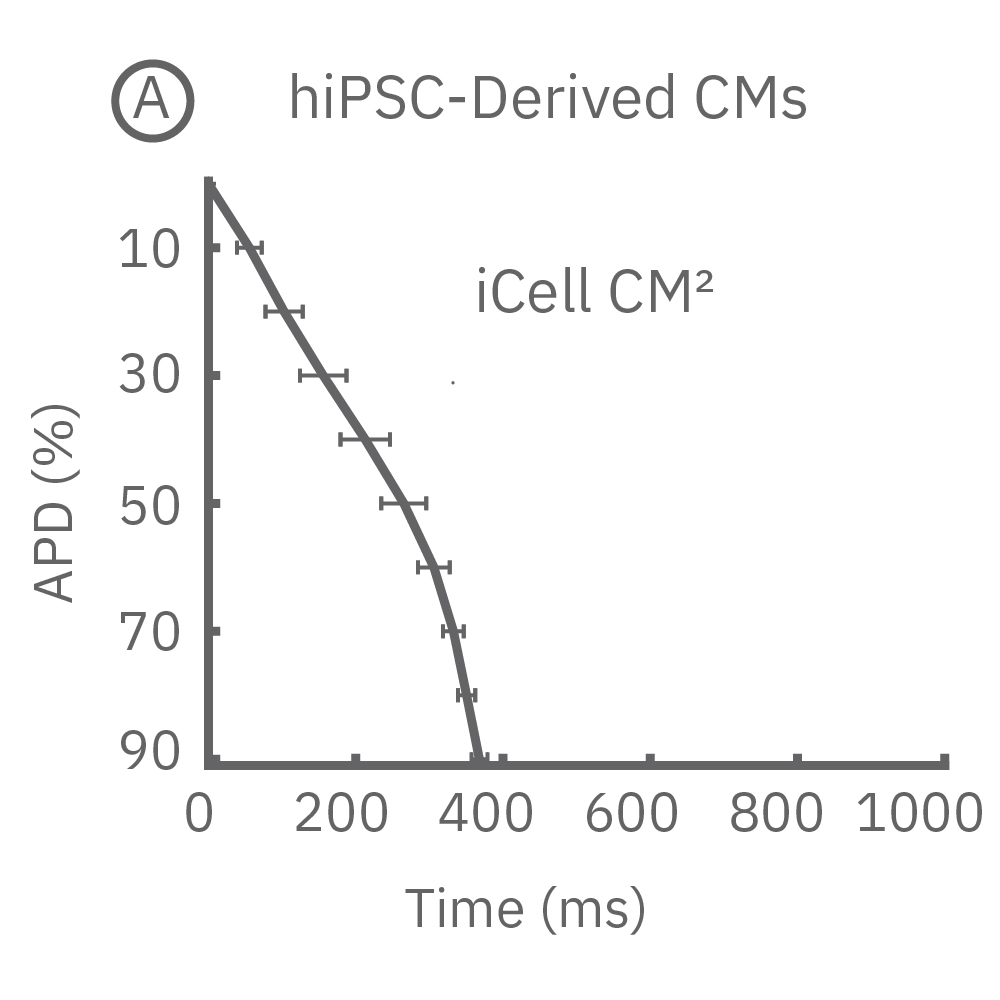
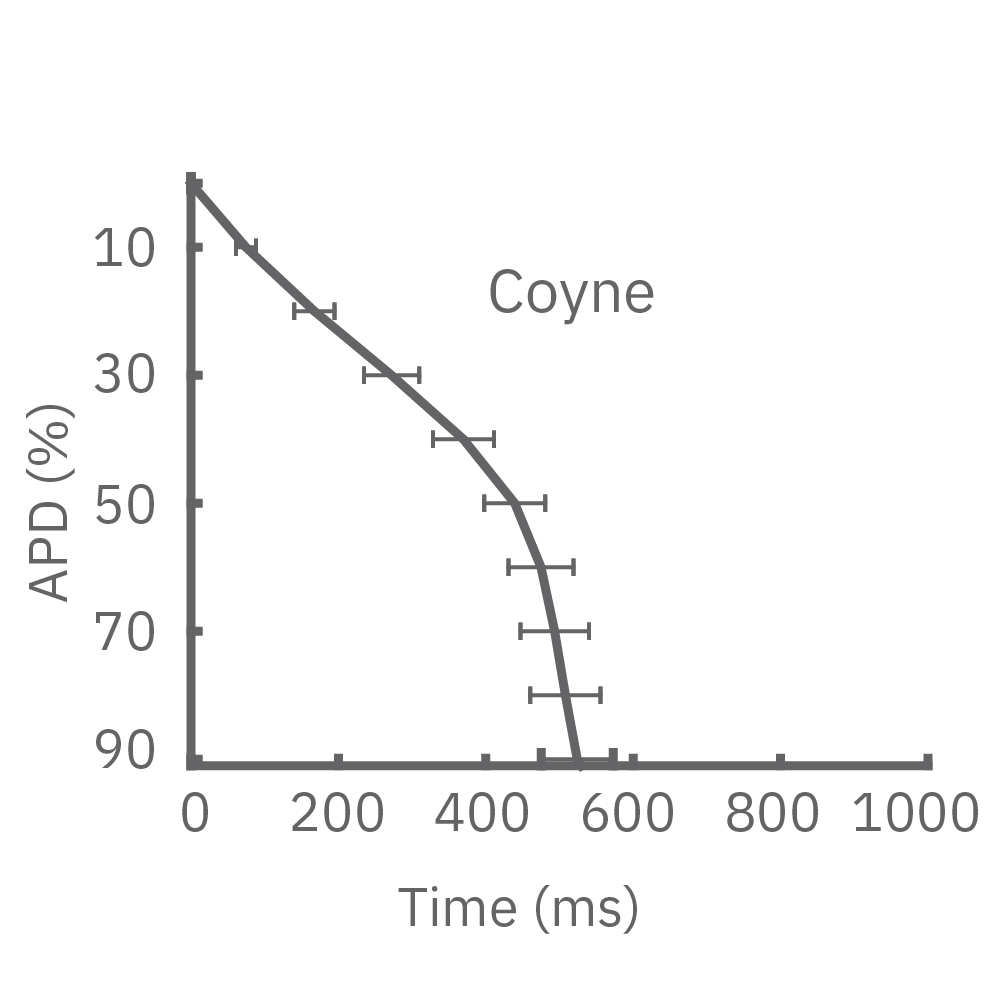
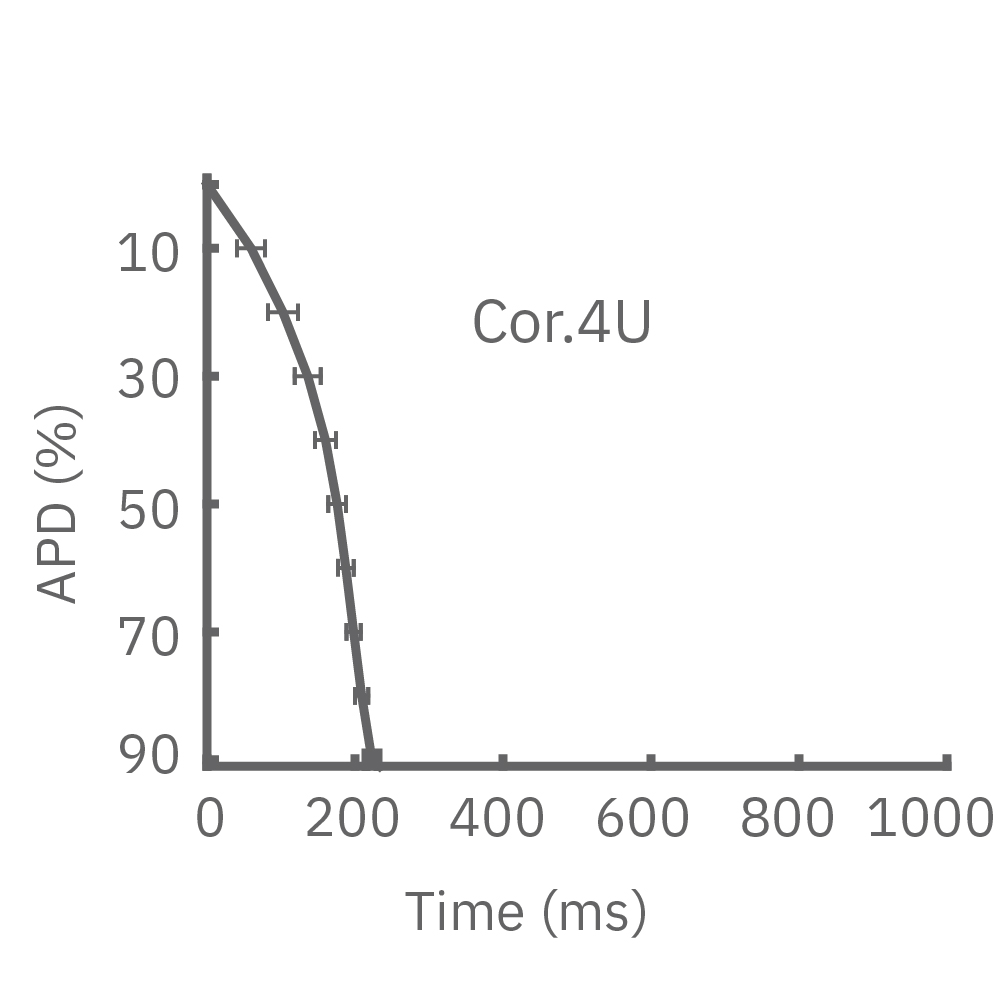
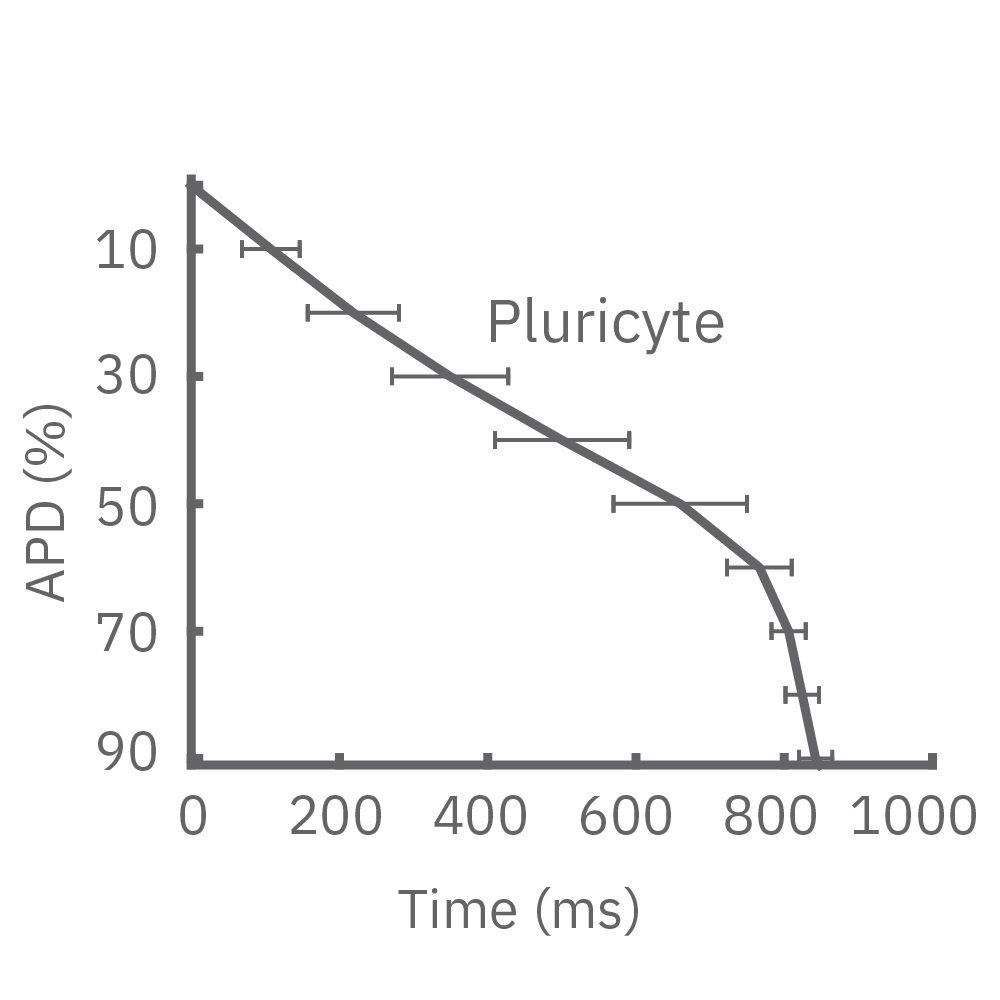
4種類のiPS細胞由来心筋細胞から得られたAPD 10-90% の平均と標準偏差を示す (iCell CM2 / n=20 wells、Cor.4U / n=17 wells、Coyne / n= 4 wells、Pluricyte / n=5 wells) 。
(A) 細胞種毎に異なる活動電位形態シグナルが得られた。APDはCor.4Uが最も短く、Pluricyte が最も長かった。ほとんどの細胞種において、活動電位終焉部の再分極フェーズにのばらつきが少なかったことから、APD90が再分極タイムポイントの有用な指標となることが示された。一方で、Coyne 心筋細胞では、再分極フェーズと比較して、プラトーフェーズのばらつきが少なかった。
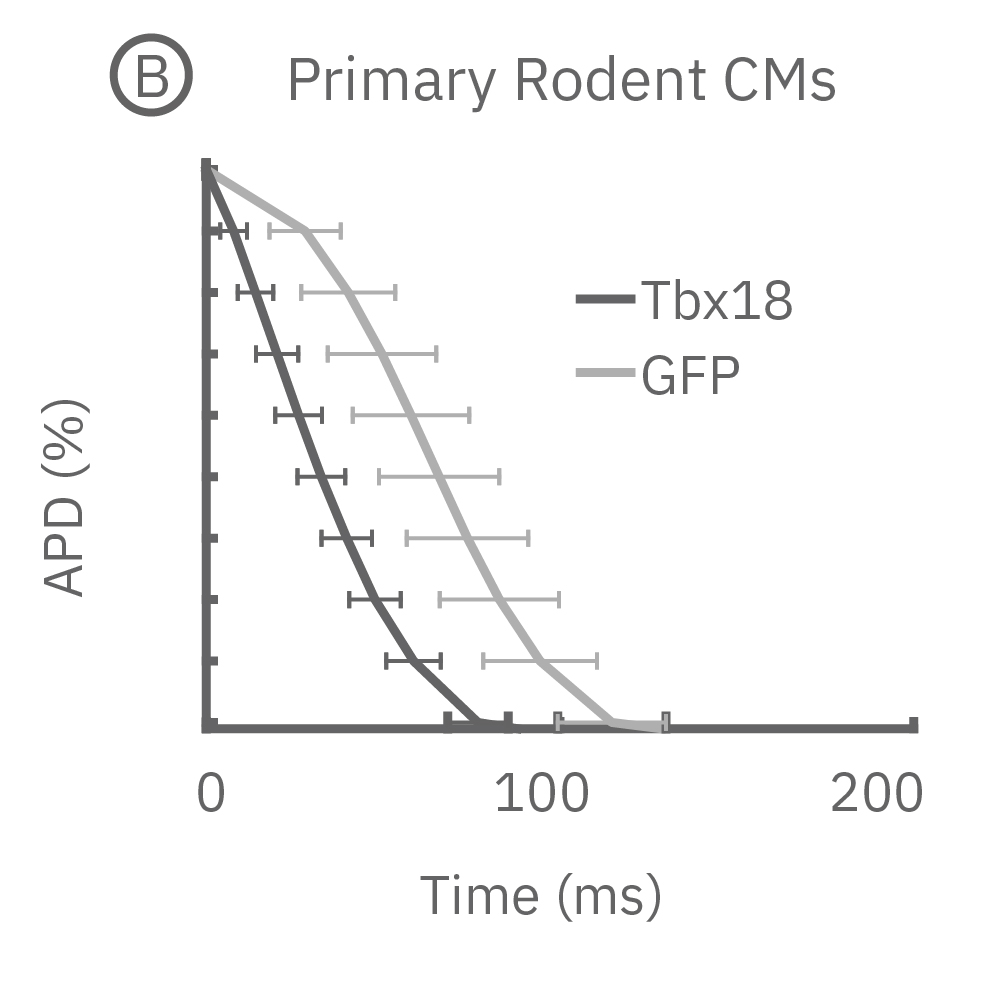
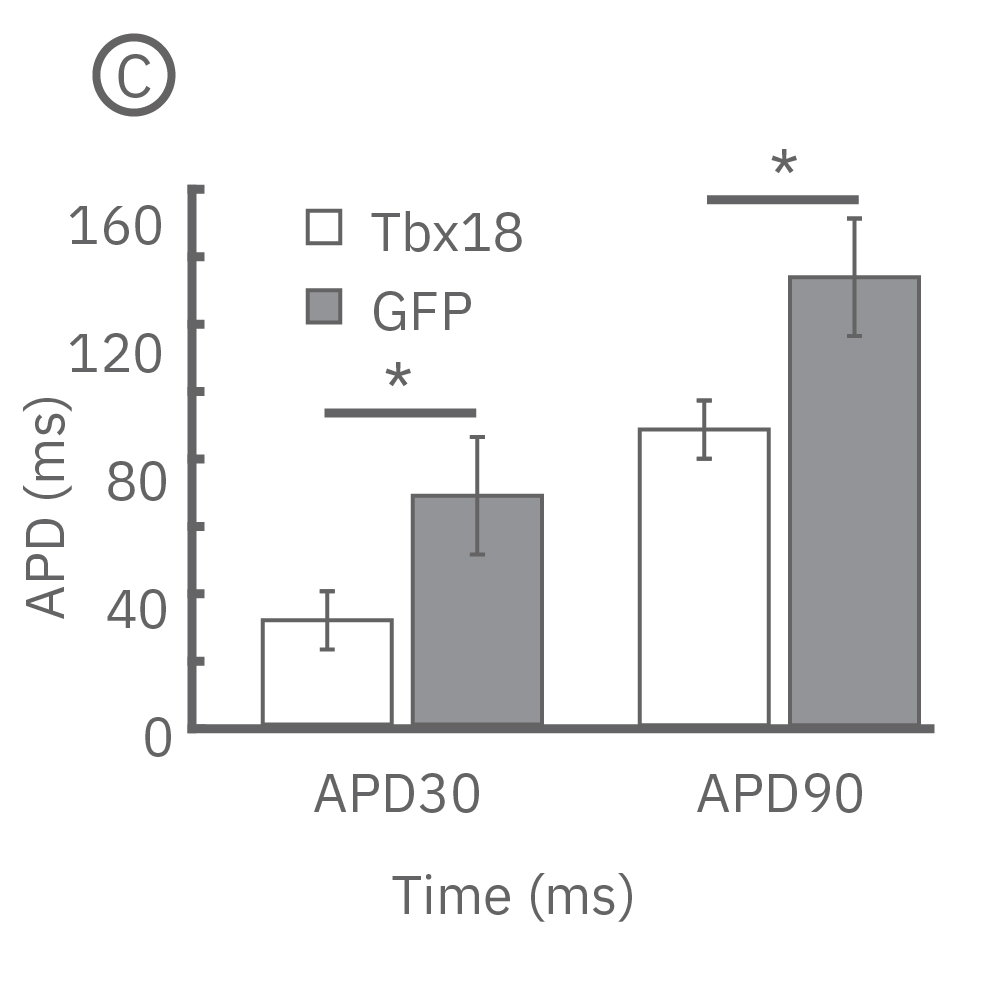
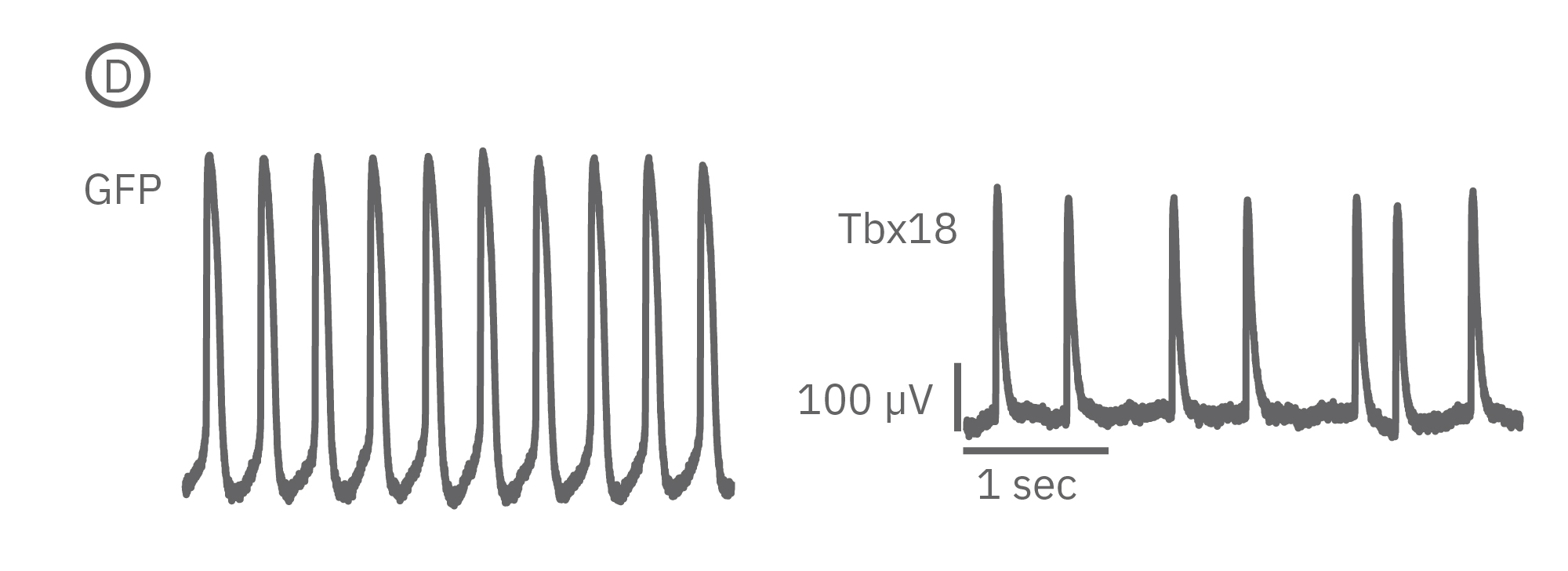
(B)新生仔ラット心室筋初代培養から測定されたLEAPシグナル。心室筋細胞をペースメーカー細胞へと再プログラミングさせる転写因子であるTbx18 導入ラット心室筋細胞と、コントロールのGFP導入ラット心室筋細胞の波形には差異が見られた 。
(C) Tbx18導入ラット心筋細胞からは、コントロールGFPと比較して、短いAPD30及びAPD90が得られた。
(D)更に、Tbx18導入ラットからは、コントロールGFPと比較して、非常に高い拍動間隔変動係数が示された。
胎児の心筋細胞は、成人の心筋細胞には無い生来の自動性を示すことが報告されていることから、このような活動電位波形の差異は、ヒトiPS細胞由来心筋細胞の未熟性によるものと推察することも可能です。また、細胞種類による拍動頻度のばらつきは、ペーシングにて拍動頻度を一定化させることの重要性を提起しています。
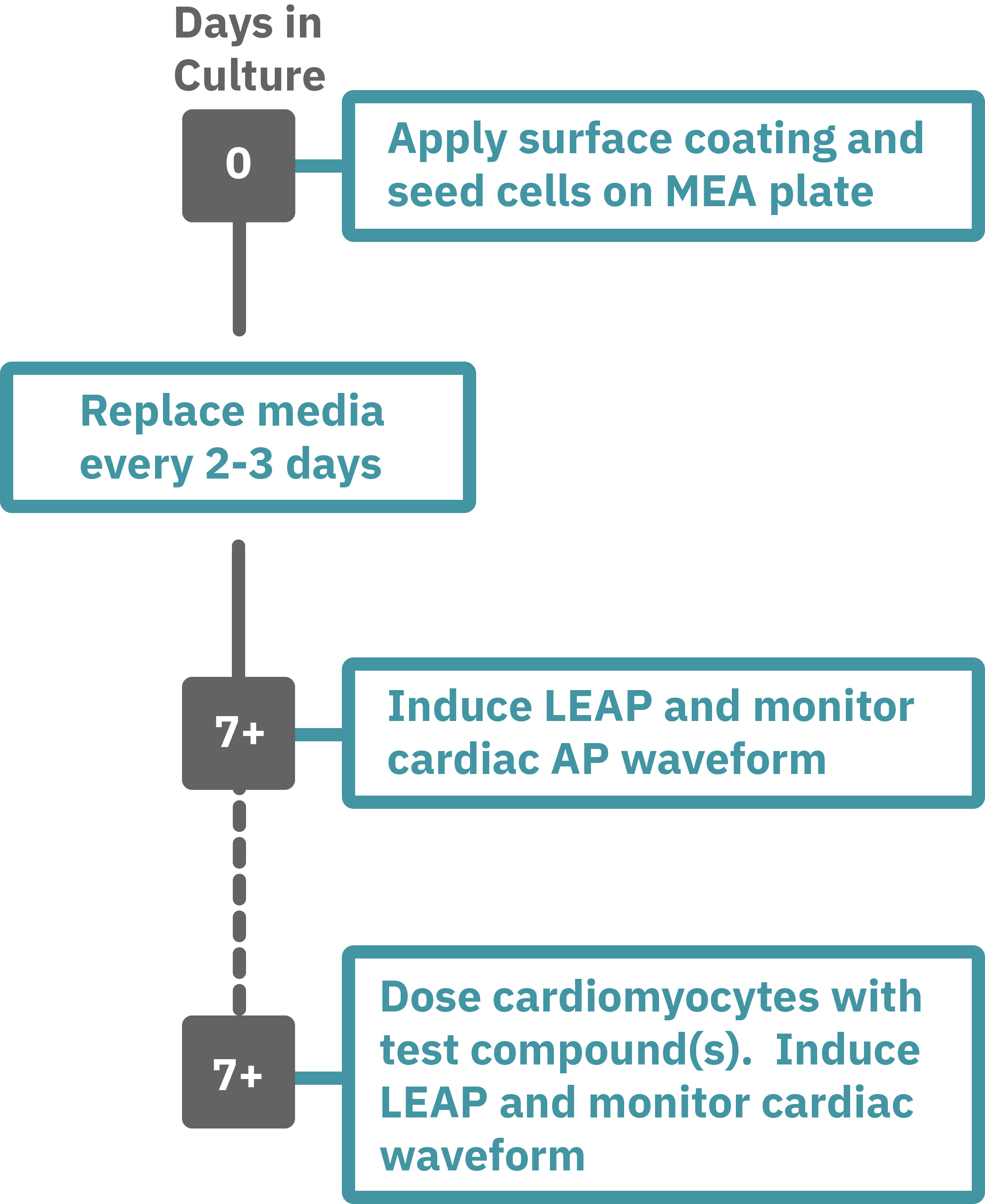
Maestro Pro/Edge による心筋細胞評価は非常に簡単です。
コーティングされたMEAプレート上に直接心筋細胞を播種します(Day 0)。2-3日毎に培地交換をしながら、一定期間細胞を培養します。
データ測定日 (例: Day 7+) にプレートを Maestro に搭載し測定を行います。データは付属のソフトで解析可能です。

Maestro MEA による心筋細胞の識別:特徴
-
活動電位形態シグナルの測定 - 平面電極を用いて活動電位形態シグナルを測定します。電極操作は不要。簡単な操作で、パッチクランデータと同様のシグナルの取得・解析が可能です。
-
1システムで4種類のアッセイ - Maestro Pro/Edgeでは、1枚のプレートで次の4種類のアッセイが行えます。[1] 細胞外電位応答 [2] シグナル伝播 [3] Contractility (弛緩収縮評価) [4] LEAP (活動電位形態シグナル)
-
ラベルフリー・リアルタイムで細胞の電気的な活動を測定 - プレート上の電極を用いて心筋細胞の活動電位を測定します。ラベルフリー、リアルタイムな測定は、試薬など2次的要因によるゆがみがなく、細胞の変化をより正確にとらえます。また、数日から数週間に渡る慢性評価にも最適です。
-
同一プレート上で培養から測定まで - MEAプレート上で直接細胞を培養し、測定します。環境要因による変化を最小限にとどめながら、細胞への負担が少ない状態で、細胞の変化を検出することができます。
-
安定した環境下での実験 - 温度・CO₂ 濃度は、装置搭載のコントローラで自動制御されます。また、Maestro は外来ノイズ・振動に影響されにくい設計になっています。安定した環境で毎日安心して実験に望めます。
-
簡単操作 - 電気生理未経験の方でも簡単に実験が行えます。MEAプレート上に細胞を培養し、装置に搭載するだけで、心筋細胞の電気的な活動の測定が可能です。付属のソフトウエアパッケージを用いて、数クリックで複数の指標に基づいた解析結果が得られ、結果の作表まで行うことができます

Cardiac MEA
Show Full DetailsWhat is a microelectrode array (MEA)?
Microelectrode arrays (MEA), also known as multielectrode arrays, contain a grid of tightly spaced electrodes embedded in the culture surface of the well. Electrically active cells, such as cardiomyocytes, are cultured on top of the electrodes. When neurons fire action potentials, the electrodes measure the extracellular voltage on a microsecond timescale. As the cells attach and connect with one another, an MEA can simultaneously sample from many locations across the culture to detect propagation and synchronization of cardiac activity across the syncytium.
That’s it, an electrode and your cells. No dyes, no incubation steps, no perfusion, no positioning things just-so; just your cells in a well. Because the electrodes are extracellular (they do not poke into the cells), the recording is noninvasive and does not alter the behavior of the cells, you can measure the activity of your culture for seconds or even months!
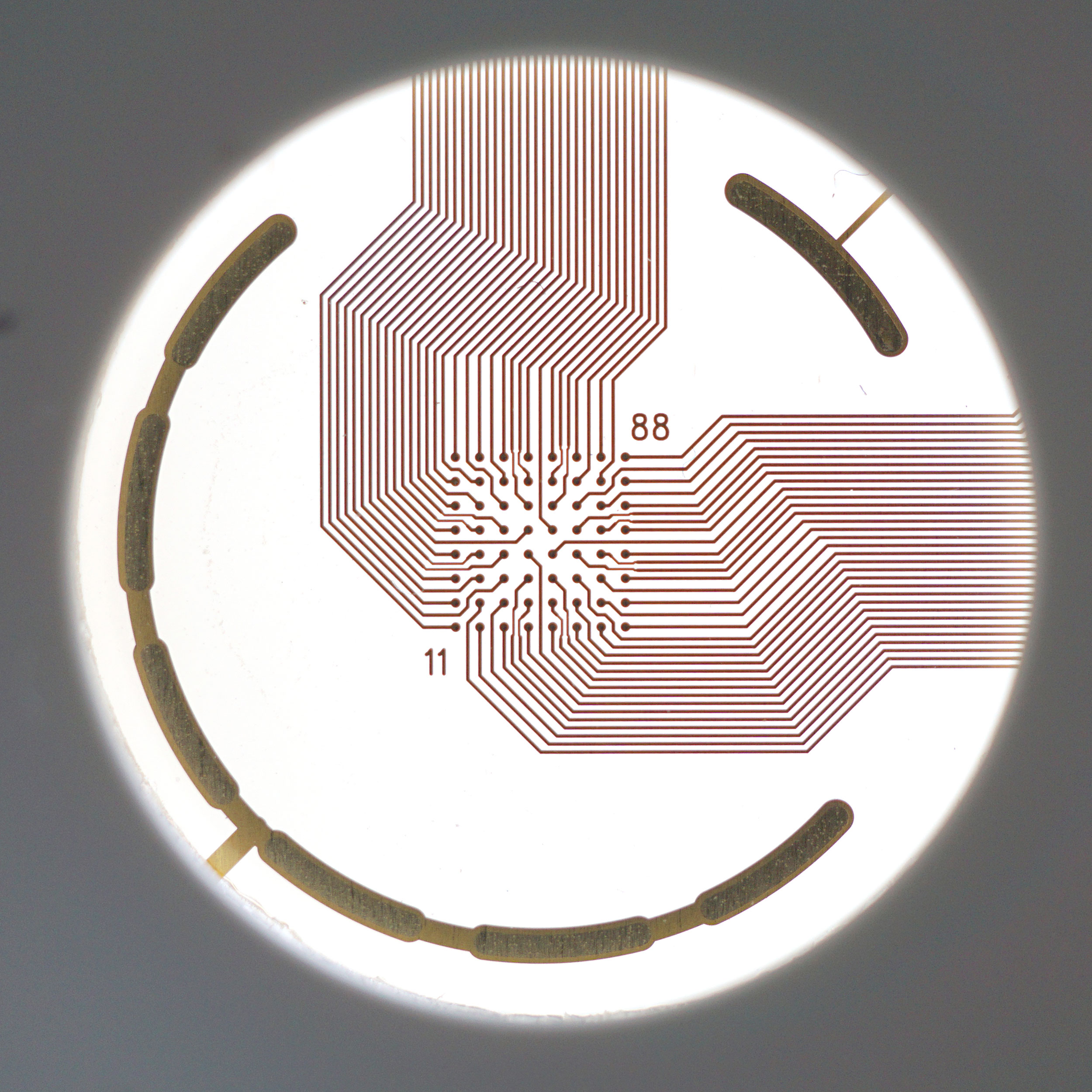
An MEA of 64 electrodes embedded in the substate at the bottom of a well.
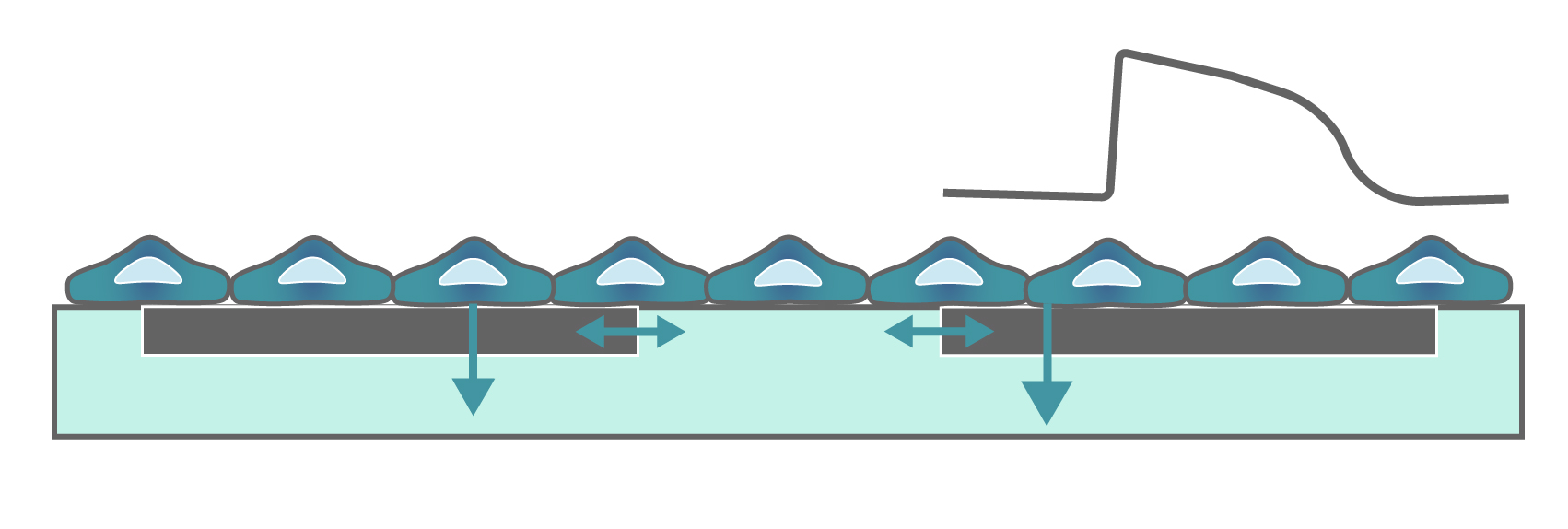
Cardiomyocytes attach to the array and form a network. The microelectrodes detect the action potentials fired as well as their propagation across the network.
Heartbeats in a dish
When cardiomyocytes are cultured on top of an MEA, they attach and connect to form a spontaneously beating sheet of cells, called a syncytium. When one cardiomyocyte fires an action potential, the electrical activity propagates across the syncytium causing each cell to fire and then contract. The electrodes detect each individual action potential and contraction, as well as the propagation of this activity across the array.
The propagating electrical signal is detected by the electrodes as an extracellular field potential. The field potential derives from the underlying cardiac action potential, but more closely resembles a clinical electrocardiogram (ECG) signal. The initial depolarization phase is seen as a sharp spike, similar to the QRS complex, and the slow repolarization is seen as a small slow spike, like a T-wave. The time from the depolarization to repolarization is termed the field potential duration (FPD) and is a key metric in predictive cardiotoxicity screening assays.
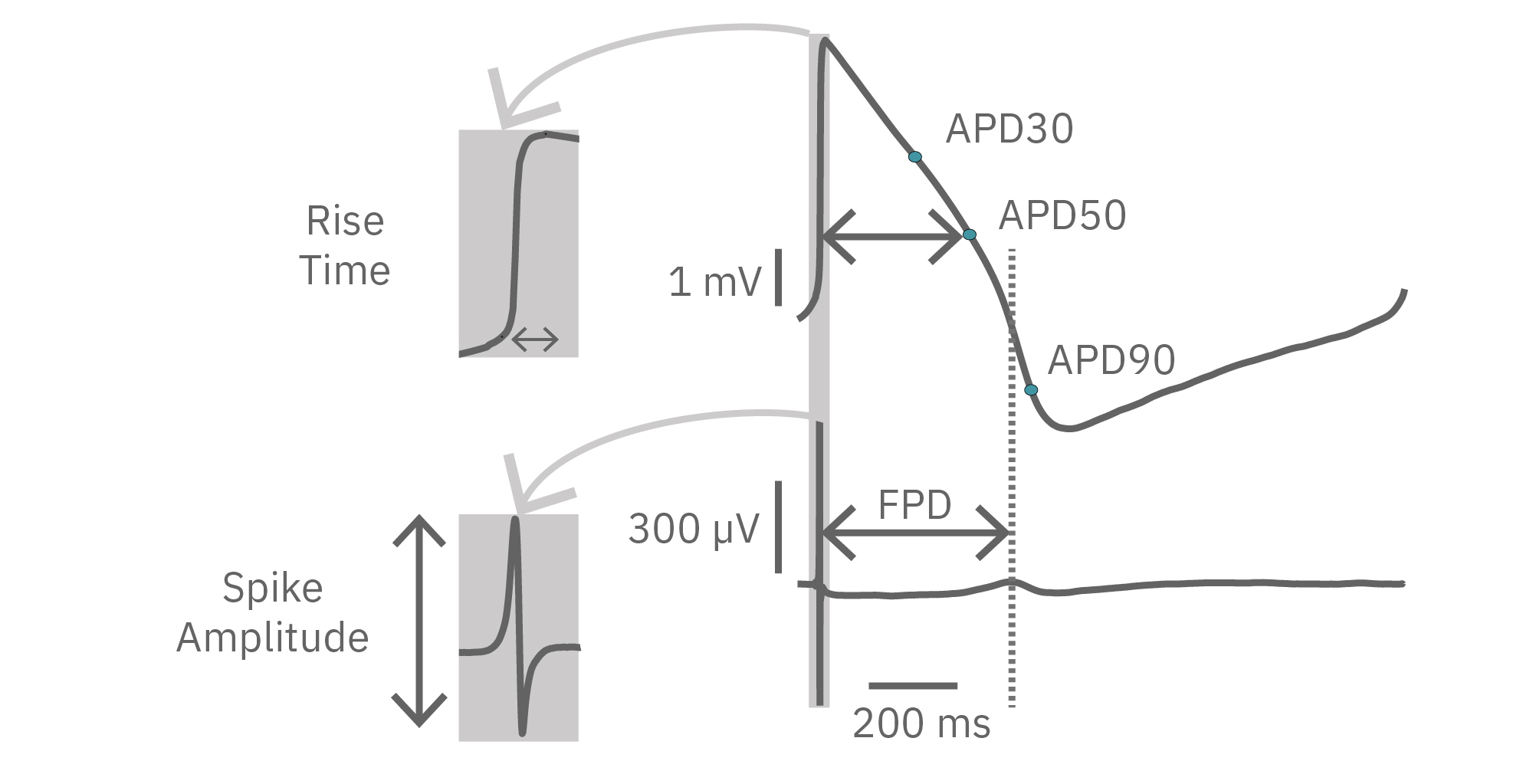
While most record the cardiac field potential, the Maestro Pro and Edge MEA systems can also measure local extracellular action potentials, or LEAP. LEAP induction increases the coupling between the microelectrodes and the cardiomyocytes, transforming the extracellular signal from a field potential to an action potential. LEAP provides additional and complementary metrics such as rise time, action potential duration (APD), triangulation, and automated early after depolarization (EAD) detection.
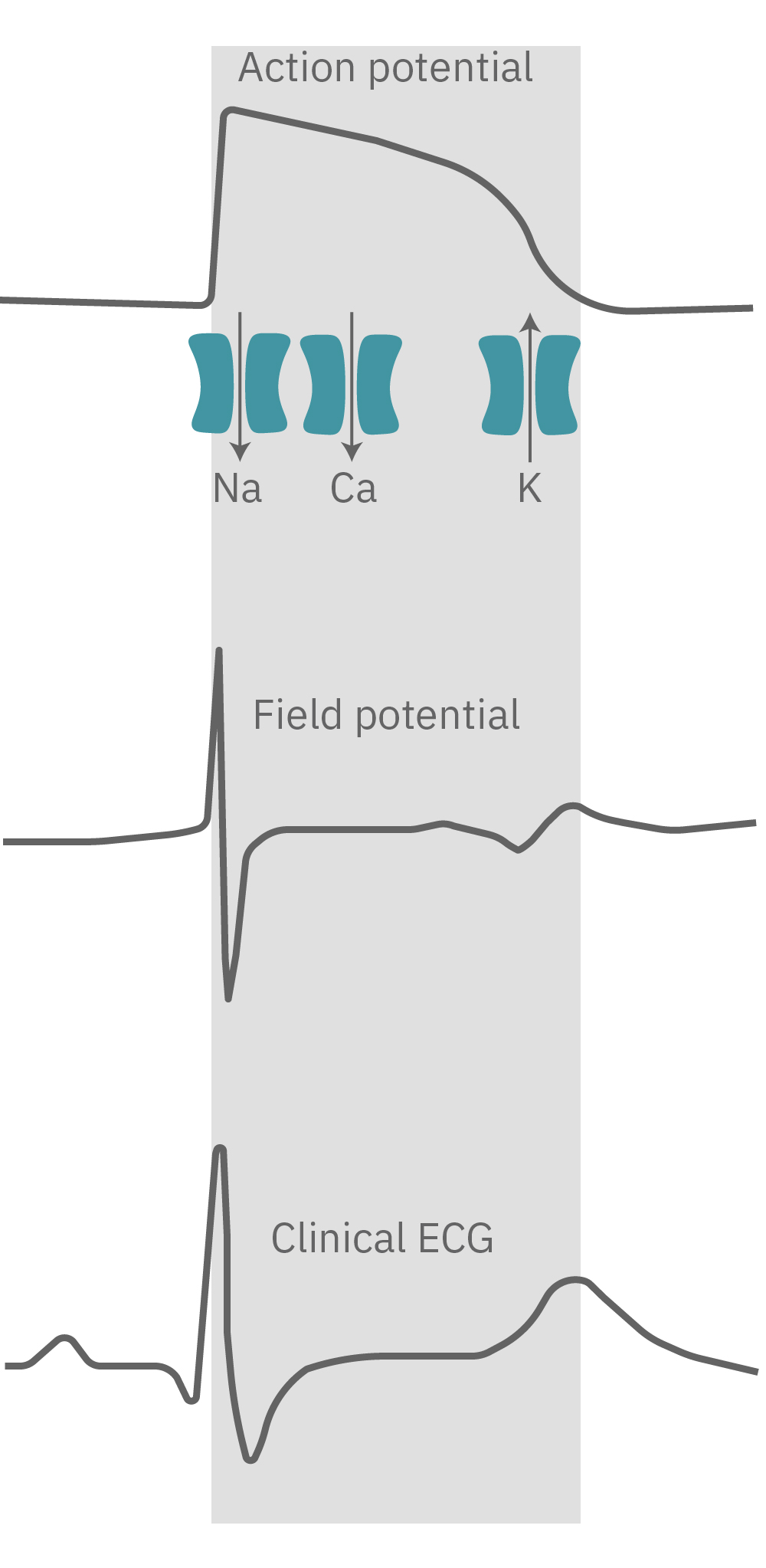
The cardiac action potential propagates from cell to cell across the syncytium. The MEA detects this activity as an extracellular field potential, which closely resembles the clinical ECG.
Do more with multiwell
Axion BioSystems offers multiwell plates at many throughputs, from 6-wells to 96-wells, with an MEA embedded in the bottom of each well. Each well represents its own unique cell culture and conditions, creating up to 96 experiments on one plate. Multiwell MEA allows you to study complex human biology in a dish, from a single cell firing to network activity, across many conditions and cell types at once.

Cardiac LEAP
Show Full DetailsLEAP: For cardiac action potential recordings
The cardiac action potential is an electrical signal characterized by the depolarization across the cell membrane of cardiomyocytes, resulting in contraction of the heart. The shapes of an action potential provide vital information about the cardiomyocyte biology, health, and response to a drug. However, measuring the cardiac action potential traditionally requires invasive techniques, such as manual patch clamp, or labels, such as voltage-sensitive dyes.
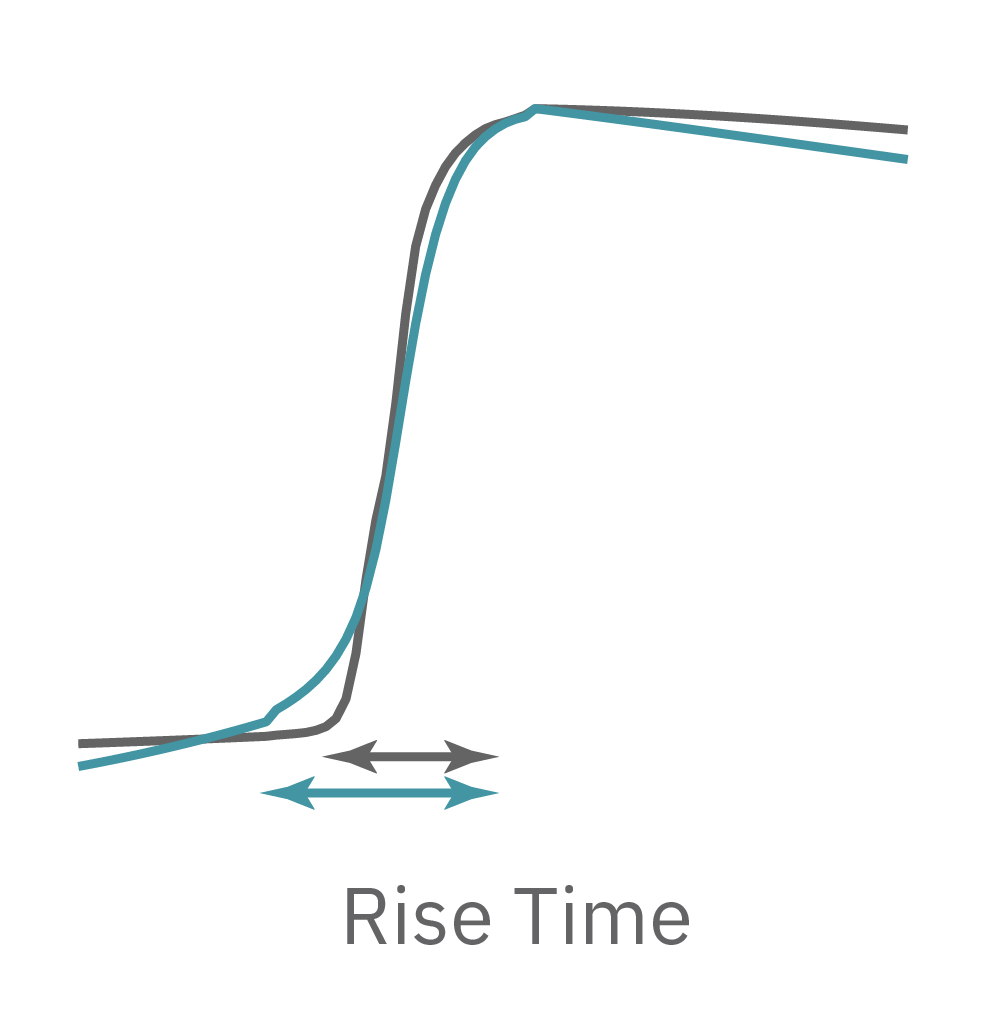
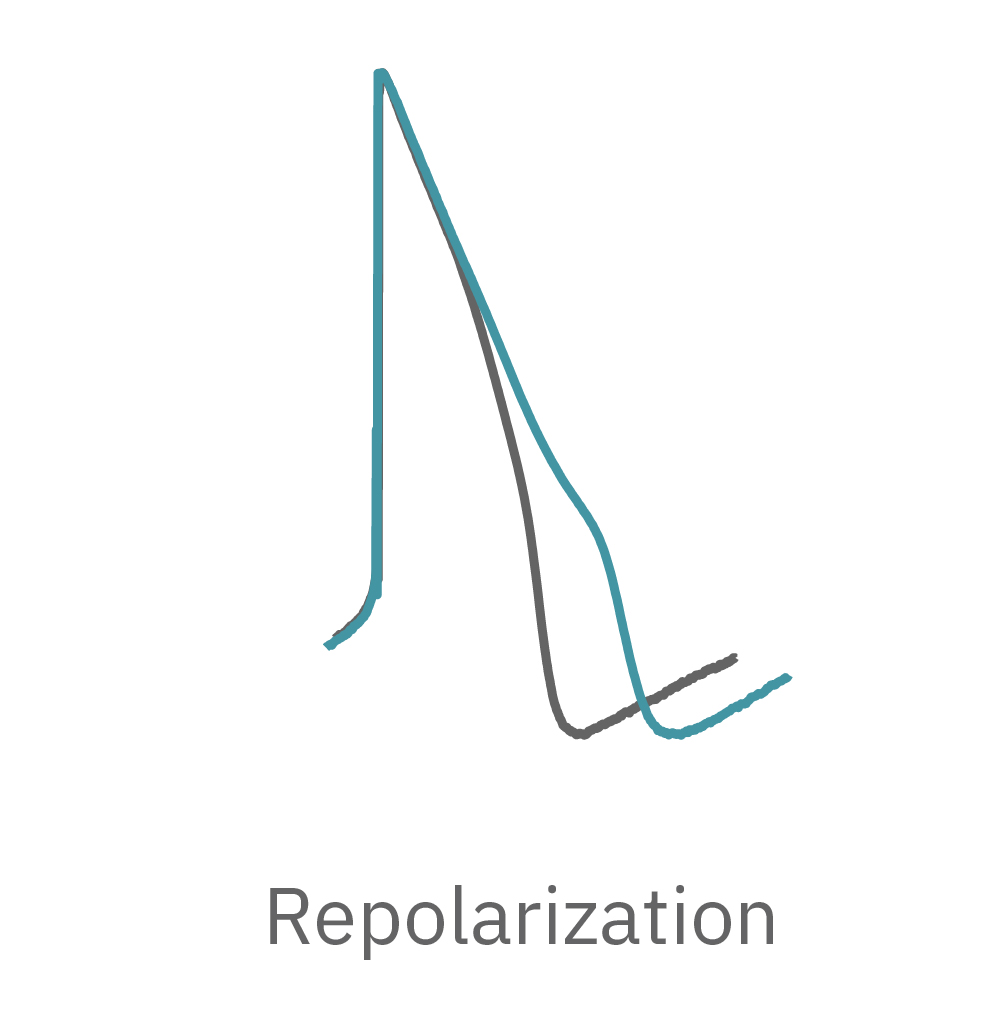
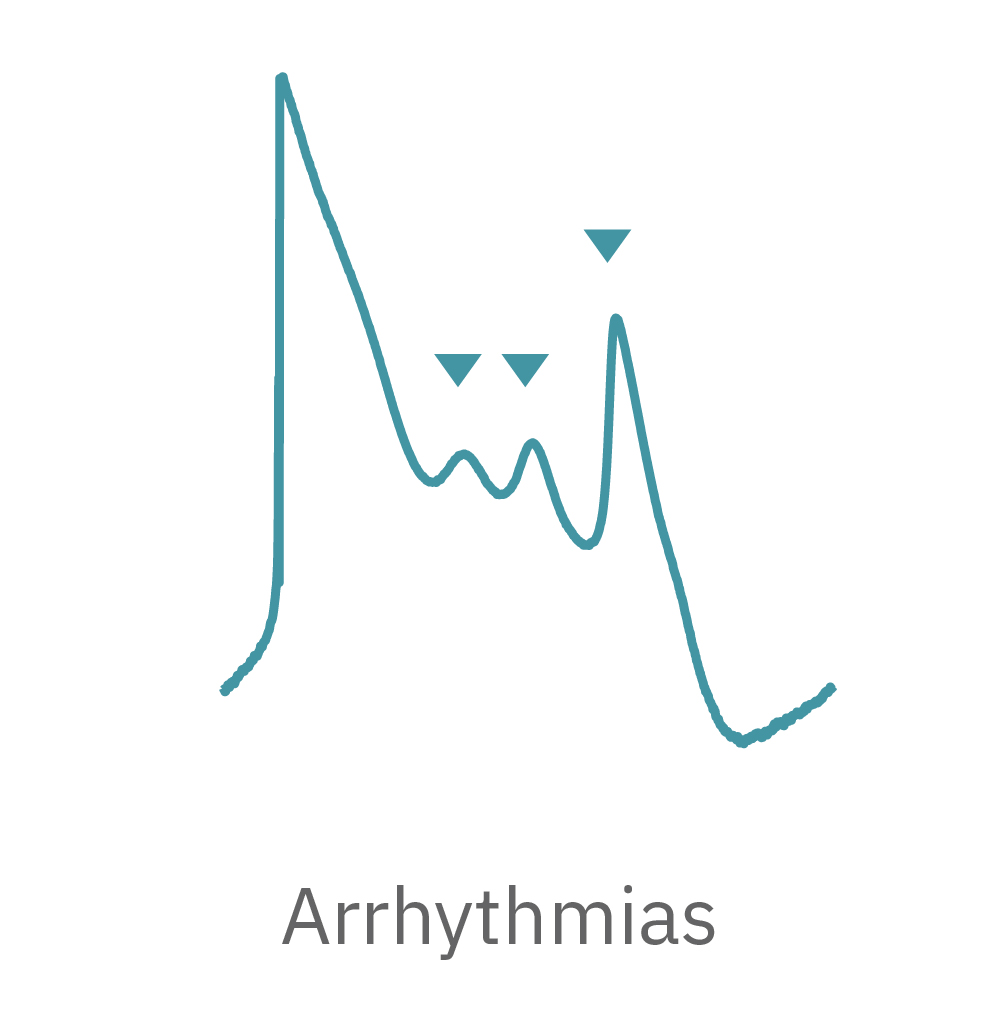
LEAP technology enables non-invasive, label-free monitoring of the cardiac action potential in a high-throughput real-time format. LEAP can be used for quantification of action potential morphology, repolarization irregularities such early after depolarizations (EADs), and arrhythmic risk factors such as triangulation. Key metrics, such as rise time, action potential duration (APD), triangulation ratio, and percentage of beats with EADs are all automatically detected by LEAP.
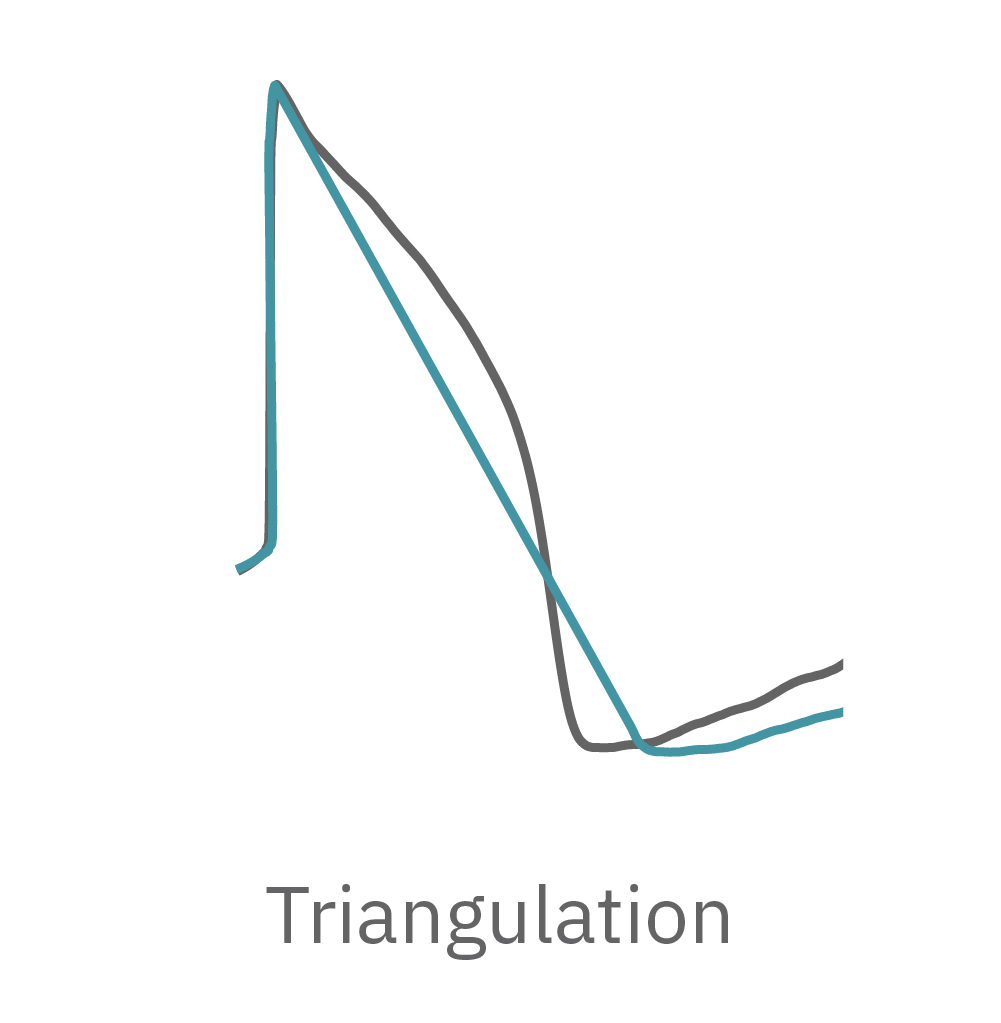
How does LEAP work?
LEAP stands for local extracellular action potential. The theory behind LEAP is similar to that of patch clamp, where the recorded signal amplitude is proportional to the sealing resistance between the electrode and the cell.
In contrast to a field potential signal, LEAP induction increases the coupling between the cells and electrode, enabling the measurement of a much larger action potential signal. The increased cell-electrode coupling is stable for 10-20 minutes or longer, allowing extracellular monitoring of the cardiac action potential without disrupting the underlying biology with dyes or invasive electrodes.
"The LEAP assay addresses an important gap in the field, namely providing a non-invasive solution in recording high quality action potentials from cardiac cells using a high throughput format. The LEAP assay may be a game changer."
Bernard Fermini, PH.D.
Chief Scientific Officer, Coyne Scientific

How does LEAP differ from the classic field potential?
The field potential has long been the standard for high-throughput in vitro cardiac electrophysiology. The field potential derives from the underlying cardiac action potential, but more closely resembles the low amplitude clinical electrocardiogram (ECG) signal. The initial depolarization phase is seen as a sharp spike, similar to the QRS complex, followed by the slow repolarization analogous to the T wave. Although the field potential has proven highly effective for high-throughput drug screening and other applications, the field potential shape can obscure complex repolarization irregularities and limit comparisons to gold standard manual patch clamp recordings.
The LEAP signal accurately reflects the shape and duration of the underlying action potential. The large signal allows for automated detection and classification of arrhythmic events, such as notched EADs, rolling EADs, or ectopic beats. LEAP also provides metrics not available from the field potential, such as rise time and triangulation.
Because LEAP operates on each electrode independently, field potential and LEAP signals can be recorded from the same well simultaneously, providing a direct mapping between features of the field potential and the action potential. In this way, LEAP enables confirmation and automation of feature detection.
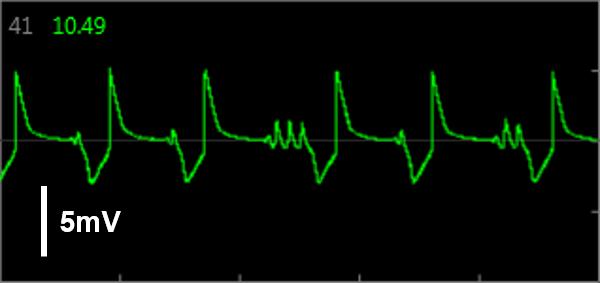
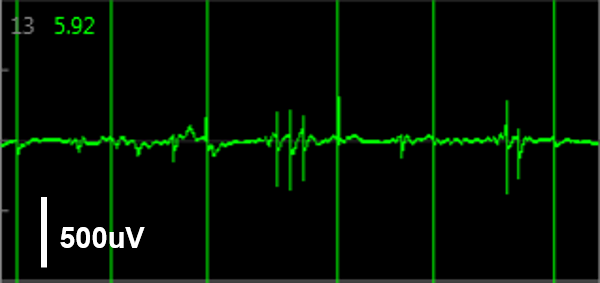
Simultaneous LEAP and field potential (FP) measurements establish translation between FP and LEAP signals. LEAP was induced on half of the microelectrodes in each well of a MEA 48-well plate of iCell CM2. Example LEAP and FP signals from the same well when dosed with E-4031. Depolarization aligned between the LEAP and FP signals, but EADs could be more reliably detected in the LEAP trace.
Advantages of LEAP
Due to the larger features of the signal, the LEAP assay is robust against pharmacological manipulations that can render field potential features difficult to detect.
For example, sodium channel blockers cause a detectable change in spike amplitude. At higher doses though, the spike amplitude can become too small to detect. However, rise time prolongation in the LEAP signal can still be easily measured even at high doses. Similar effects can be seen at high doses of hERG blockers when hERG block begins to impact the resting membrane potential.
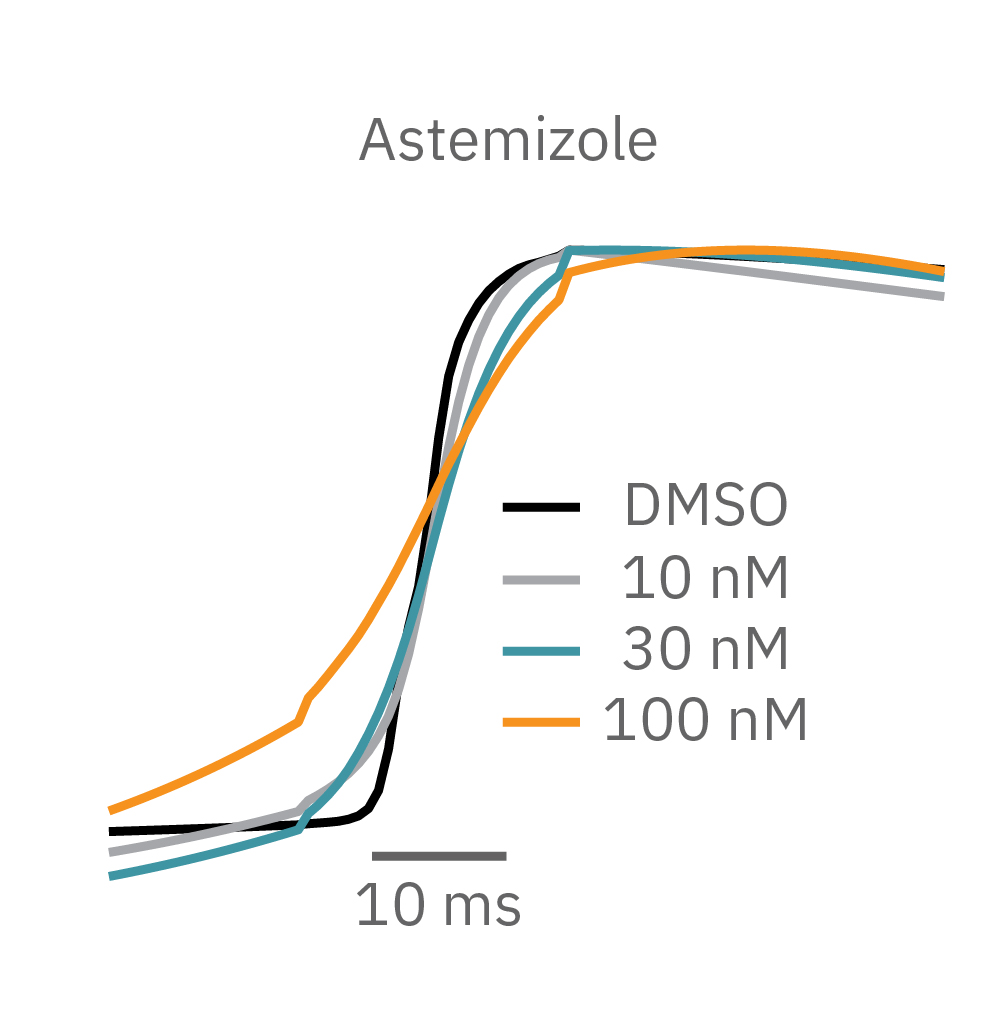
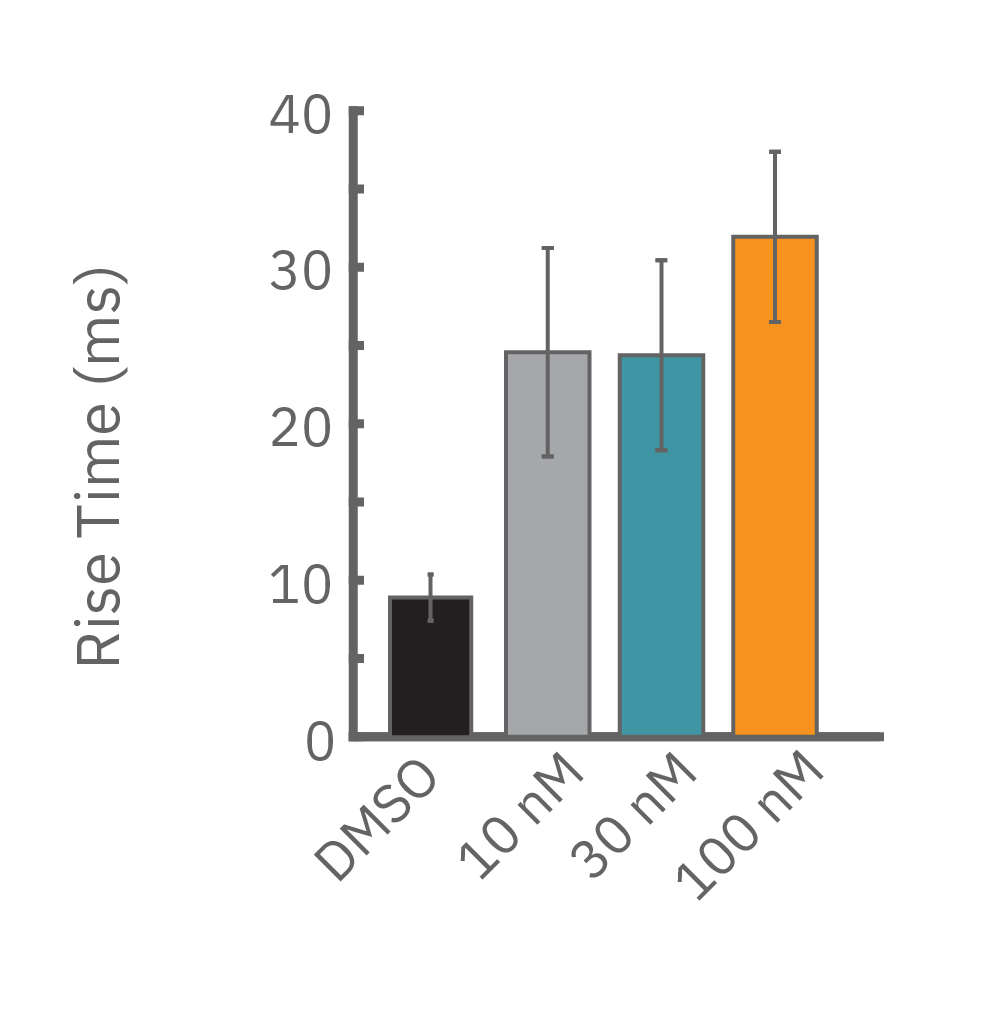
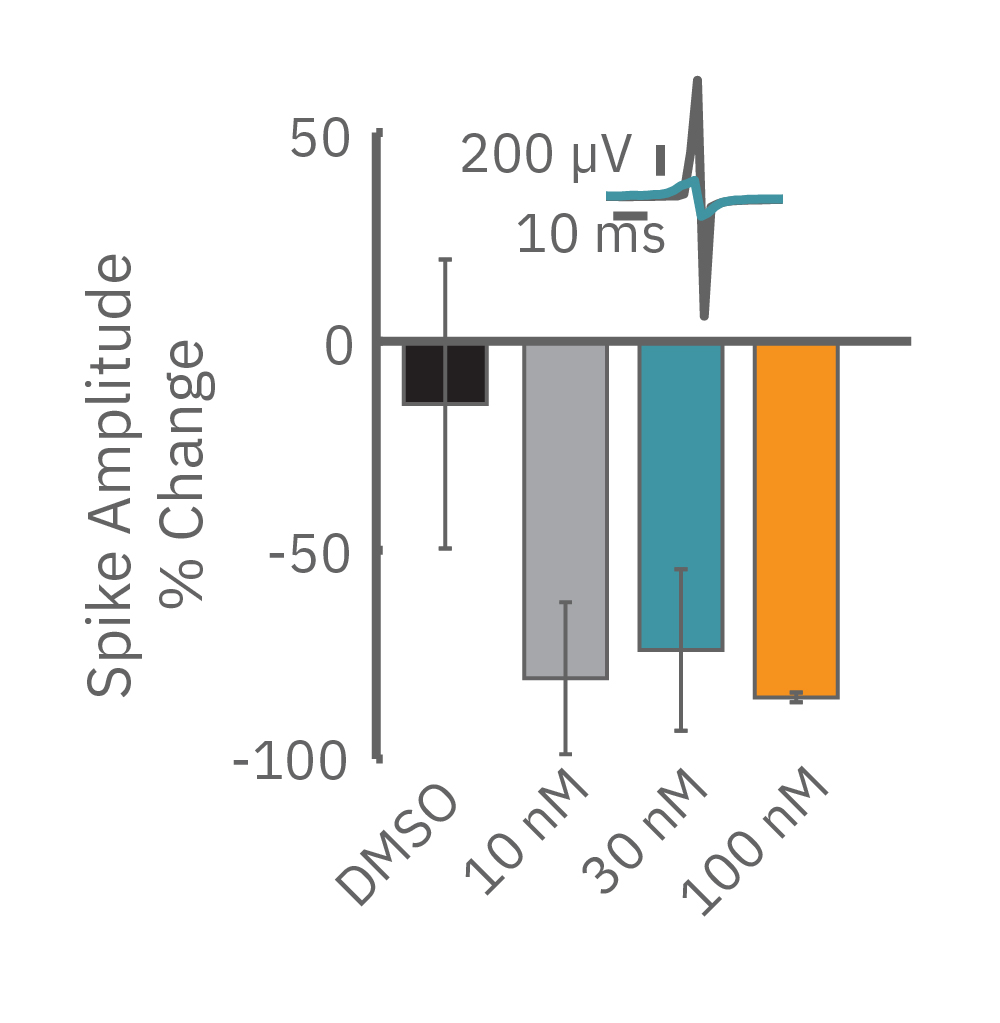
Similarly, compounds, such as terodiline, that induce triangulation can flatten the field potential repolarization feature, referred to as the “T wave”. The resulting broad, small amplitude T wave is difficult to detect and quantify. In contrast, triangulation is readily detectable and quantifiable in the LEAP signal.
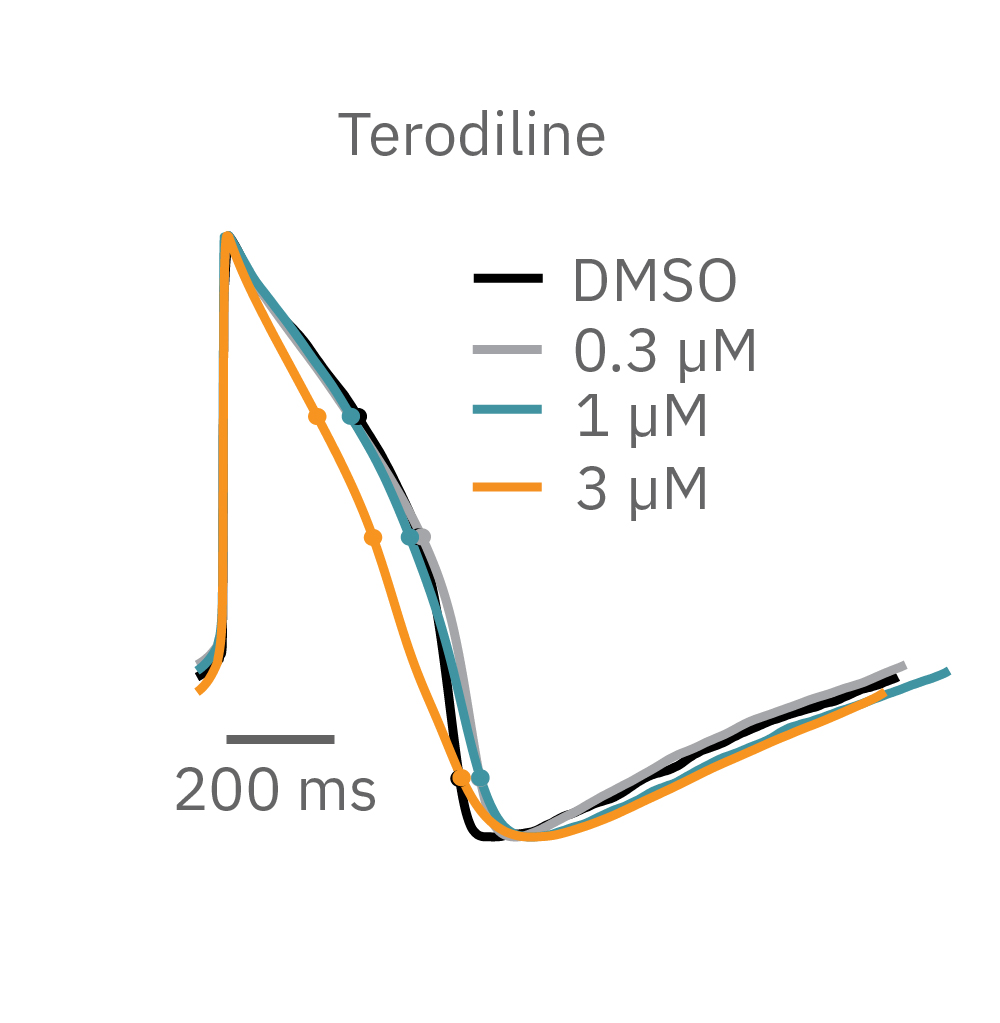
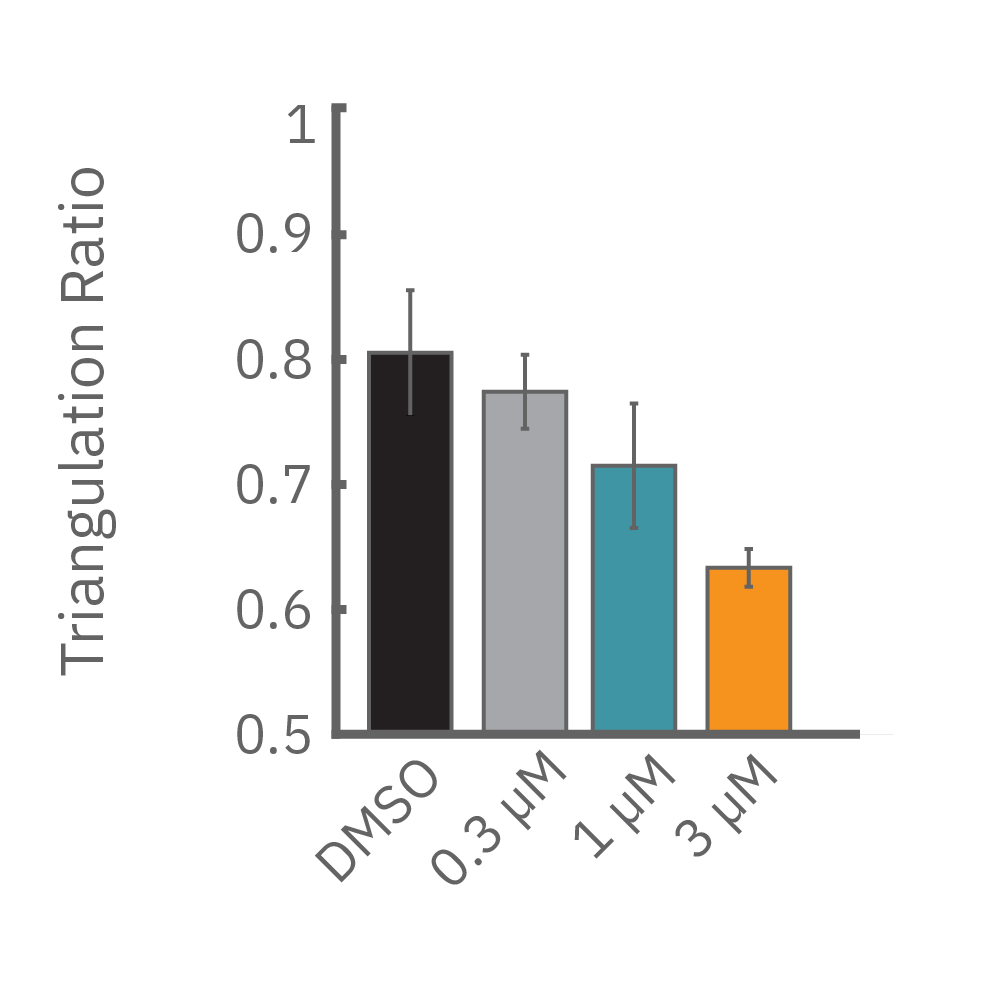
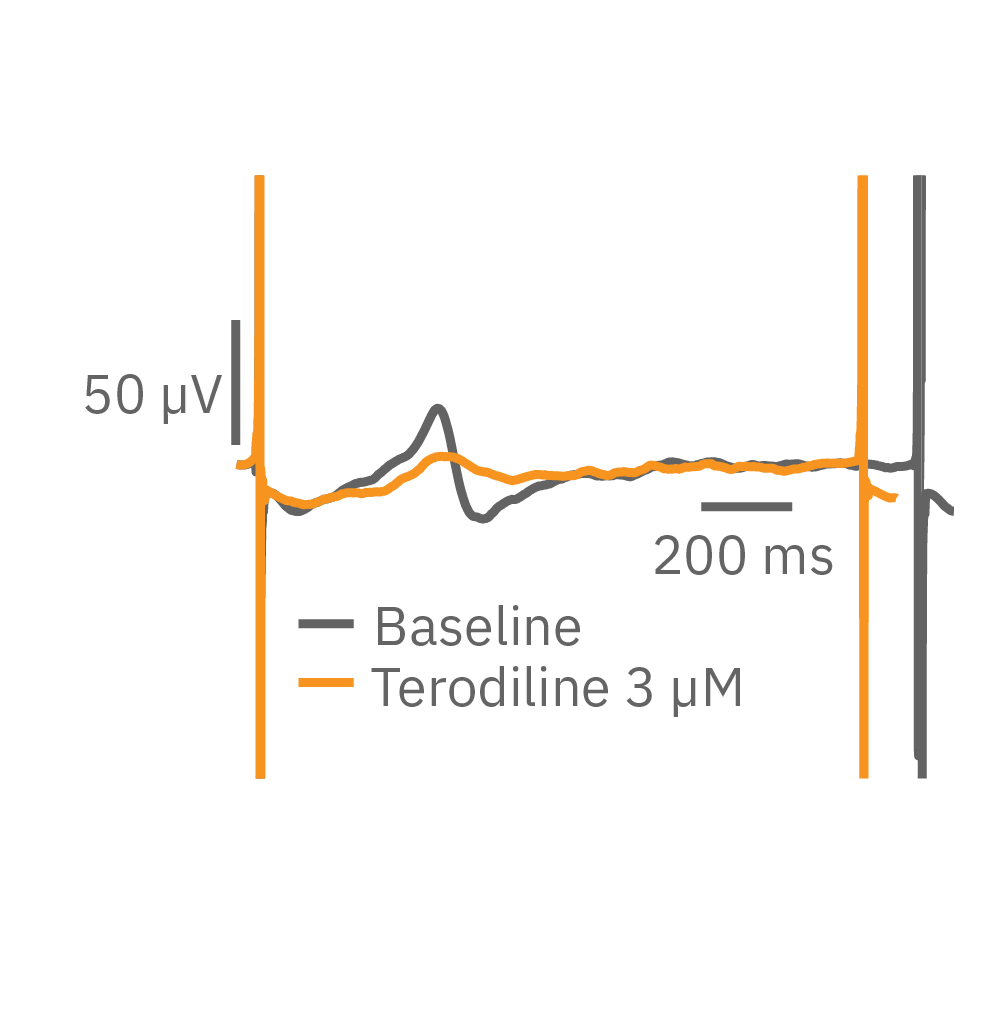
LEAP applications
LEAP is ideal for capturing even small differences in action potential morphology in cardiac health and disease and in response to compounds. LEAP is a powerful tool for many applications including:
- Automated APD and EAD detection for high throughput drug screening
- Predicting arrhythmic risk for cardiac safety and cardiotoxicity testing
- Characterization of action potential morphology in human induced pluripotent stem cell-derived (hiPSC) cardiomycoytes
- Studying the effects of genetic manipulation on cardiac electrophysiology
- Comparing cardiac biology in healthy and diseased states