How can MEA be applied in drug discovery and development for neurological diseases?
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is a fatal neurodegenerative disease of the motor nervous system. Although several factors have been implicated in the disease, including genetic mutations, protein misfolding, and neuroinflammation, it remains poorly understood how these factors impact motor neuron biology and cause neural degeneration.
Gain deeper insights into the mechanisms of ALS with Axion's live-cell platforms, like Maestro microelectrode array (MEA), for investigation of electrical activity of neurons in vitro.
Characterization of ALS
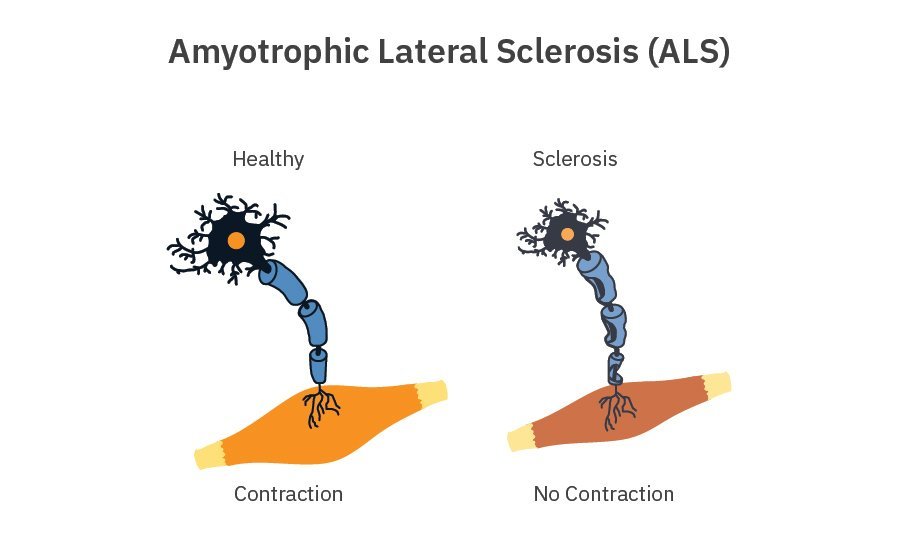
Amyotrophic lateral sclerosis can present with different clinical manifestations and progression rates and is likely influenced by both genetic and environmental factors.
The pathogenesis of ALS is complex but often characterized by:
- Motor neuron degeneration
- Formation of protein aggregates
- Oxidative stress and neuroinflammation
- Muscle atrophy and paralysis
Axion’s in vitro assay platforms, like Maestro MEA, allow researchers to observe the decline in neural function associated with the development of ALS.
Study Neuropathology of ALS Using in vitro ALS Models
-
Identify phenotypic differences of ALS motor neurons>
-
Test the efficacy of potential ALS treatments in vitro>
-
Model neuromuscular junctions and stimulate with light>
-
Publication Highlights: Amyotrophic lateral sclerosis (ALS)>
Purpose: To evaluate in vitro phenotypes of iPSC-derived ALS motor neuron models. It has previously been reported that ALS neurons demonstrated neural hyperexcitability in vivo.
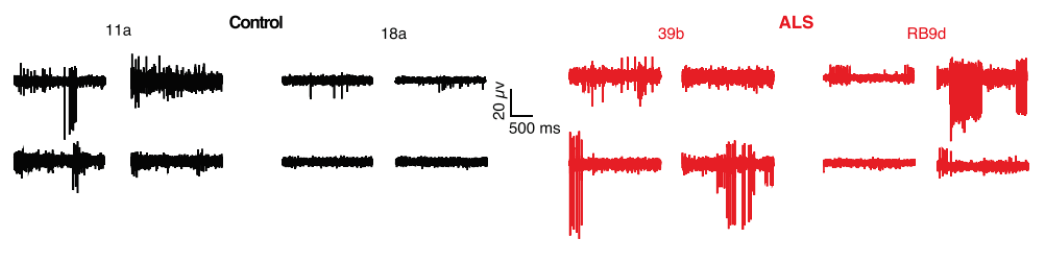
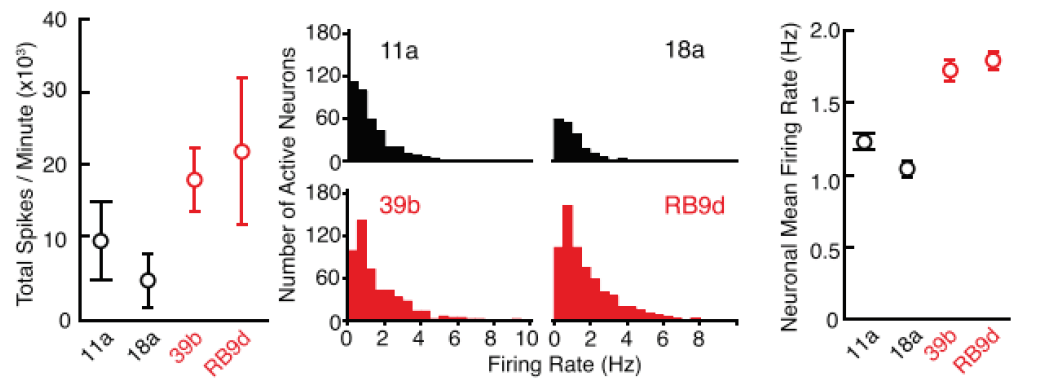
Activity of iPSC-derived ALS neurons harboring SOD1A4V/+ mutation and control cells was measured using patch clamp and the Maestro MEA platform.
Result: ALS neurons demonstrated an increase in firing in both patch clamp and MEA, suggesting that ALS neurons are more hyperexcitable compared to the control cells as observed in in vivo models. Because MEA can measure from an entire culture in higher throughput, researchers used it to confirm the phenotype across mutations. [Wainger et al. 2014].
Purpose: To validate drug screening results to reverse ALS hyperexcitability phenotype. After MEA confirmed hyperexcitability across all tested ALS-derived neurons, the researchers began to test compounds to reverse this phenotype.
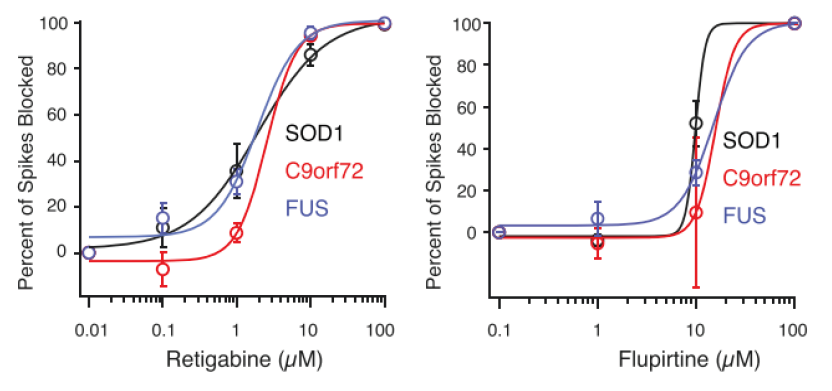
Potassium channel openers retigabine and flupirtine were tested on iPSC-derived, ALS neurons, and the firing rate was measured on the Maestro MEA platform.
Result: Both drugs were found to reduce ALS-related hyperexcitability. As a result of these studies, Retigabine entered into clinical trial as a potential treatment for ALS. [Wainger et al. 2014].
Purpose: to create an in vitro model of the neuromuscular junction (NMJ). The failure of the NMJ is a key component of degenerative neuromuscular diseases, including ALS. A co-culture model may offer patient-specific significant insights into pathogenic mechanisms that underlie NMJ dysfunction in disease.
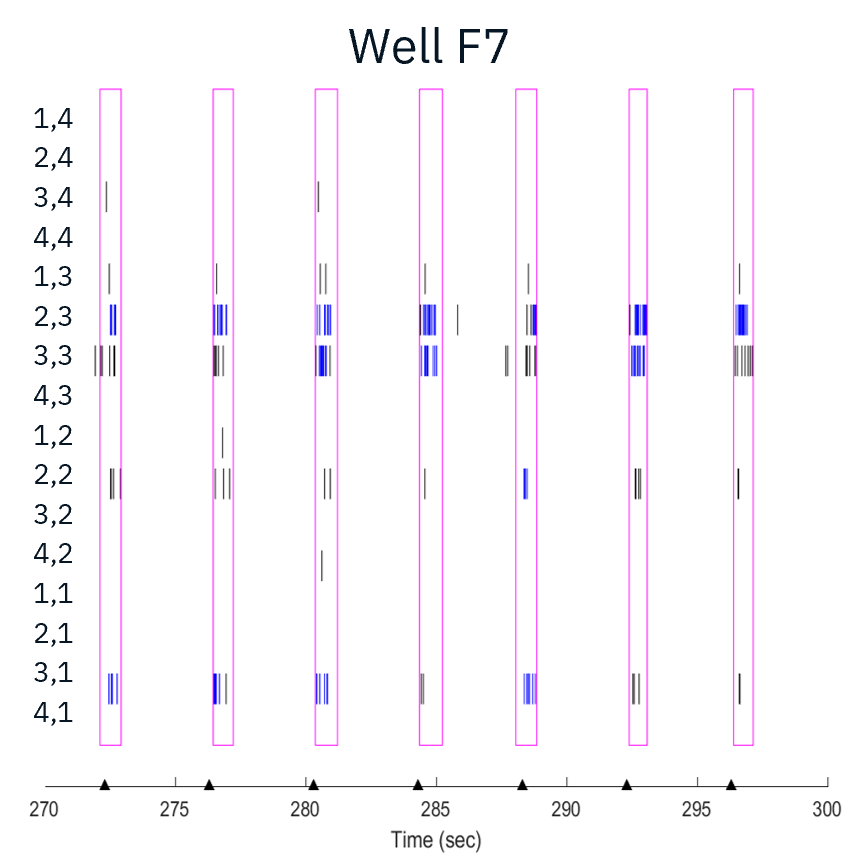
Channel rhodopsin (ChR2) expressing motor neurons were cocultured with skeletal myotubes. Motor neurons were able to elicit muscle contractions when stimulated with blue light using the Lumos.
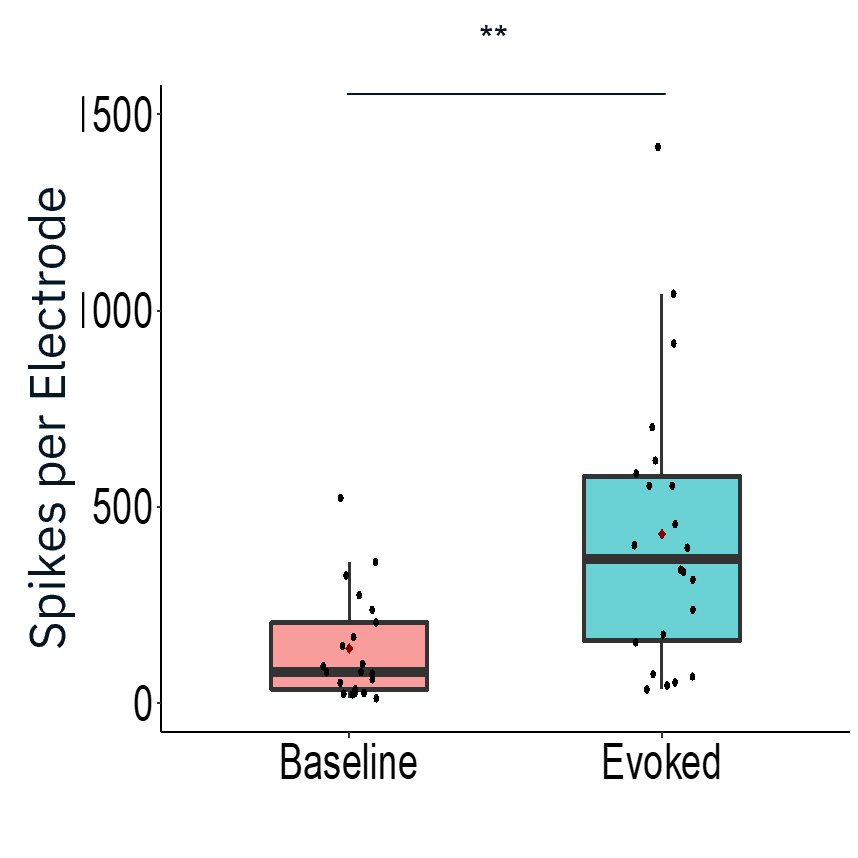
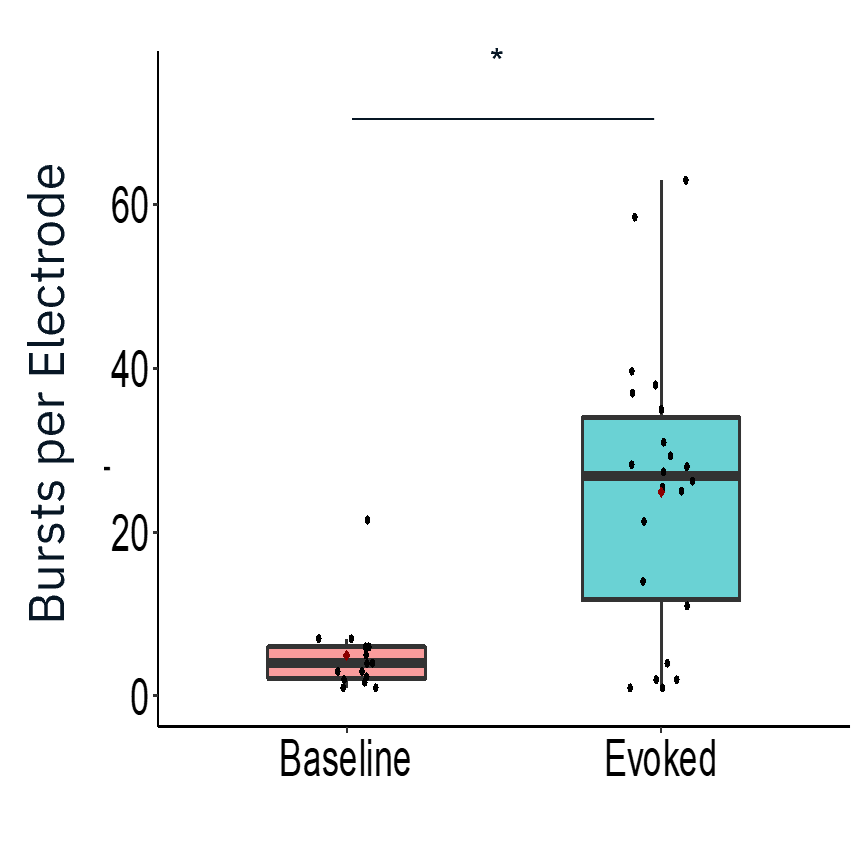
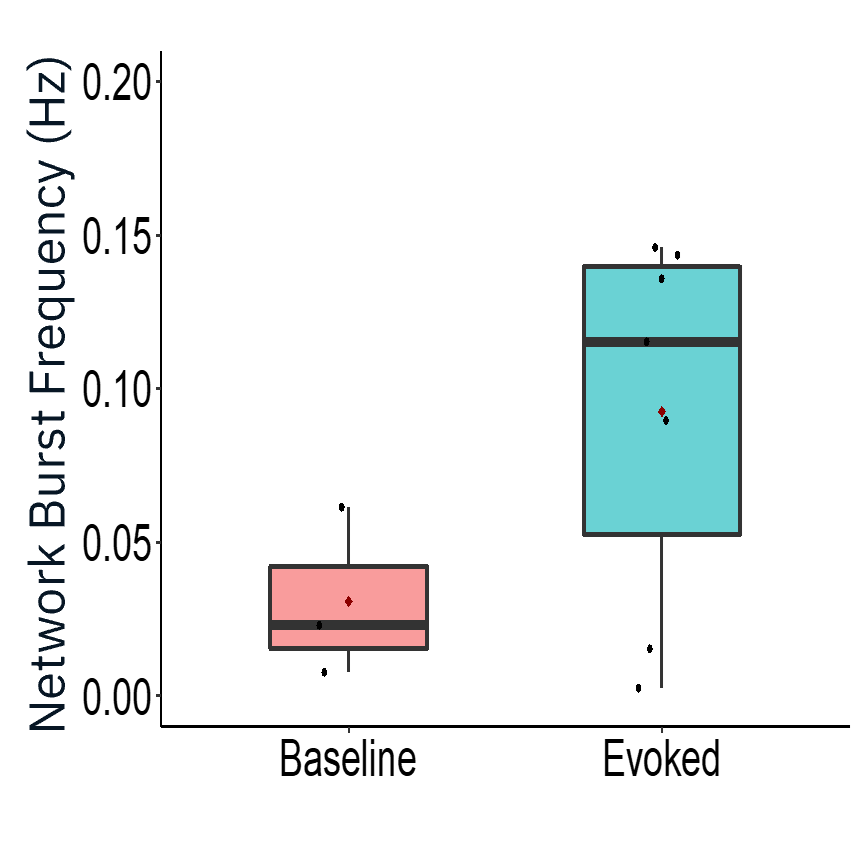
Characterization of evoked firing activity using the Maestro Pro showed robust evoked activity.
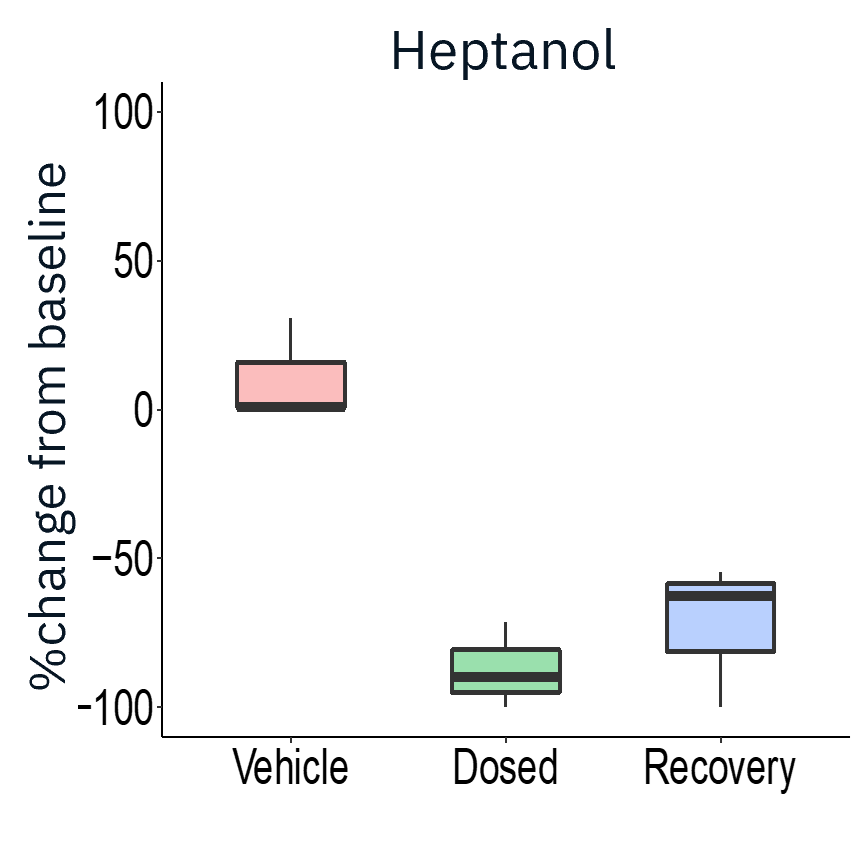
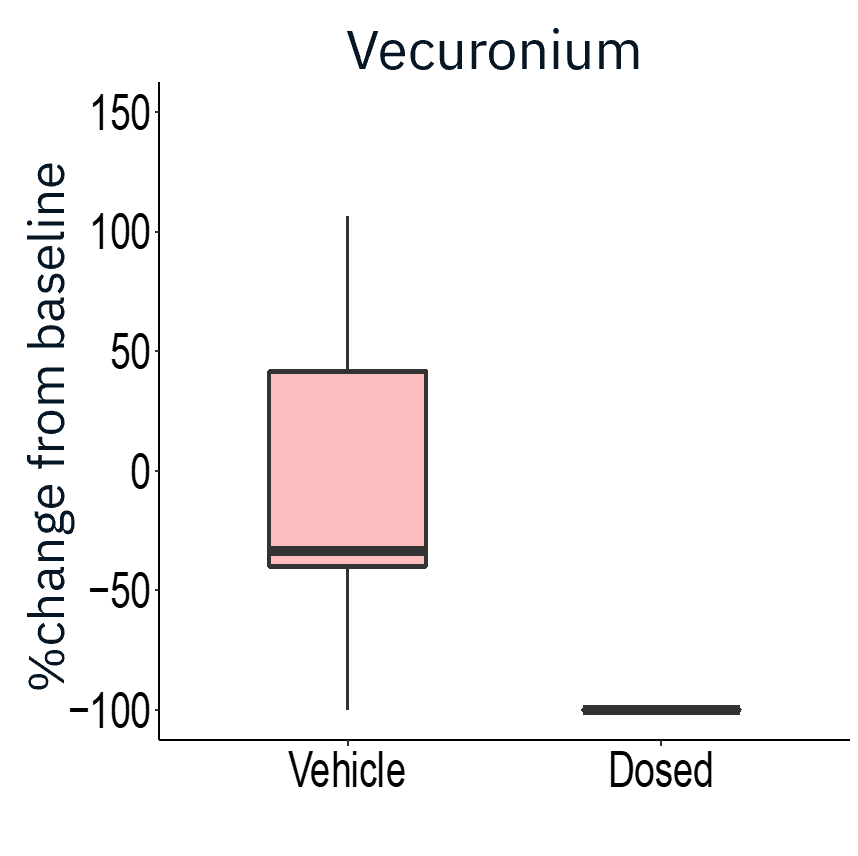
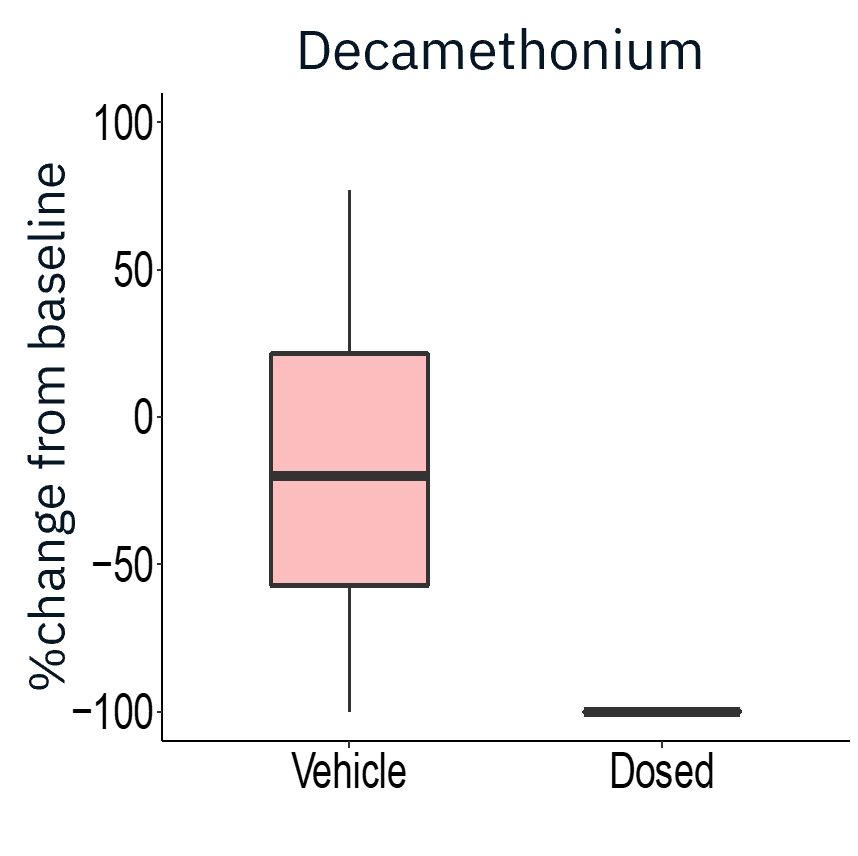
The application of gap junction blocker heptanol or neuromuscular antagonists decamethonium bromide and vecuronium drastically reduced evoked activity, demonstrating that activity measured was primarily from skeletal myotubes.
Result: The Maestro MEA platform could selectively stimulate motor neurons which activated skeletal myotubes, demonstrating a functional neuromuscular junction that responded appropriately to compounds.

Publication Highlights: Amyotrophic lateral sclerosis (ALS)
Learn how Axion's live-cell platforms were used in ALS research with these selected publications.
FAQ:
MEA is employed in drug screening to assess the effects of potential therapeutics on neural network function in a high-throughput manner. Researchers can use MEA to evaluate candidates for their ability to modulate neural activity, restore normal network function, or counteract disease-related abnormalities. This information is crucial for identifying promising drug candidates early in the development process.
What are the advantages of MEA to study in vitro ALS disease models?
- The Maestro MEA platform offers a controlled environment for studying detailed neural network activity in vitro.
- High-throughput multi-well plates make it ideal for screening patient-specific lines and therapeutics.
- Noninvasive monitoring allows for the study of long-term effects and disease progression.
- It is easy to use, requiring only basic cell culturing techniques to measure neural electrophysiology.
What kind of metrics can you get from neural activity?
For MEA measures from multiple areas of a culture over time, allowing you to go beyond just the firing of individual neurons and evaluate dynamic network activity and the development of functional phenotypes. Learn more about what you can do with our Neural Module.
What kind of neural cultures can be measured on the Maestro MEA?
MEA is compatible with primary or stem cell-derived neurons. Neural activity can be measured from 2D cultures, organoids or other 3D cultures, and slices. To study the role of glial cells in disease, neural cocultures are frequently used.




