心筋細胞の収縮力は、変力作用によって増減します。陽性変力作用は心筋の収縮力を増大させ、陰性変力作用は減少させます。これらの陽陰性変力作用は、心臓血管系の活動において重要な役割を担います。
MEAプレート上で培養された心筋細胞は、自発的な拍動を有し、電極上で弛緩収縮の動きを繰り返します。Maestro Pro/Edgeの [Contractility] 機能は、この弛緩収縮による心筋細胞の形状の変化を、電極との接着面におけるインピーダンスの変化としてとらえます。
Maestro Pro/Edgeは、同一電極から、細胞外電位測定とインピーダンス変化の両測定が可能です。電気的な活動と弛緩収縮の両評価により、心筋細胞の機能をより深く理解することができます。
Force-frequency 応答による心筋細胞成熟化の検証
ヒトiPS細胞由来心筋細胞 (hiPSC-CMs) は、カルシウムハンドリング、収縮・弛緩等の機能においてその未成熟さが議論されています。一方で、電気的或いは機械的な刺激は、細胞を成熟化させる⼿段の1つとして有効性が⽰されています。本事例では、Maestro MEAを用いて48時間の連続ペーシングを行い、成熟の指標の1つとされる Force-frequency relationship を検証しました。
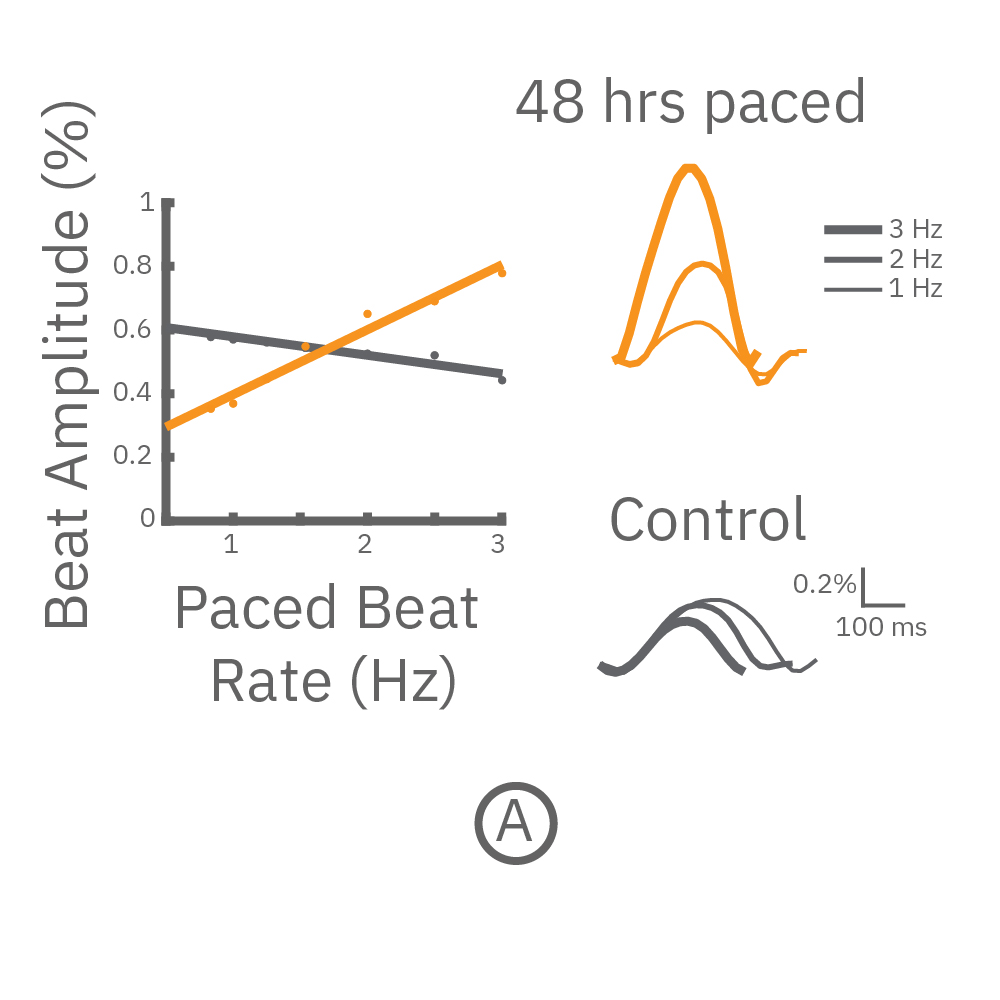
(A) CytoView 24 well MEA プレート上に iCell CM² (富士フィルムCDI)を培養し、⼀定の成熟後(DIV7-10) に2Hzのペーシングを48時間連続して印加した。ペーシング終了後、1〜3Hzのペーシングを連続印加しForce-frequency relationship を検証した。48時間連続ペーシングした iCell CM²では、刺激頻度の上昇と共に、拍動振幅値 (Beat Amplitude) の上昇が得られた (オレンジ色)。⼀⽅、Control (連続ペーシング無) の細胞では拍動振幅値は減少した(グレー色) 。右図は、1Hz、2Hz、3Hzペーシング印加時の波形を示す。
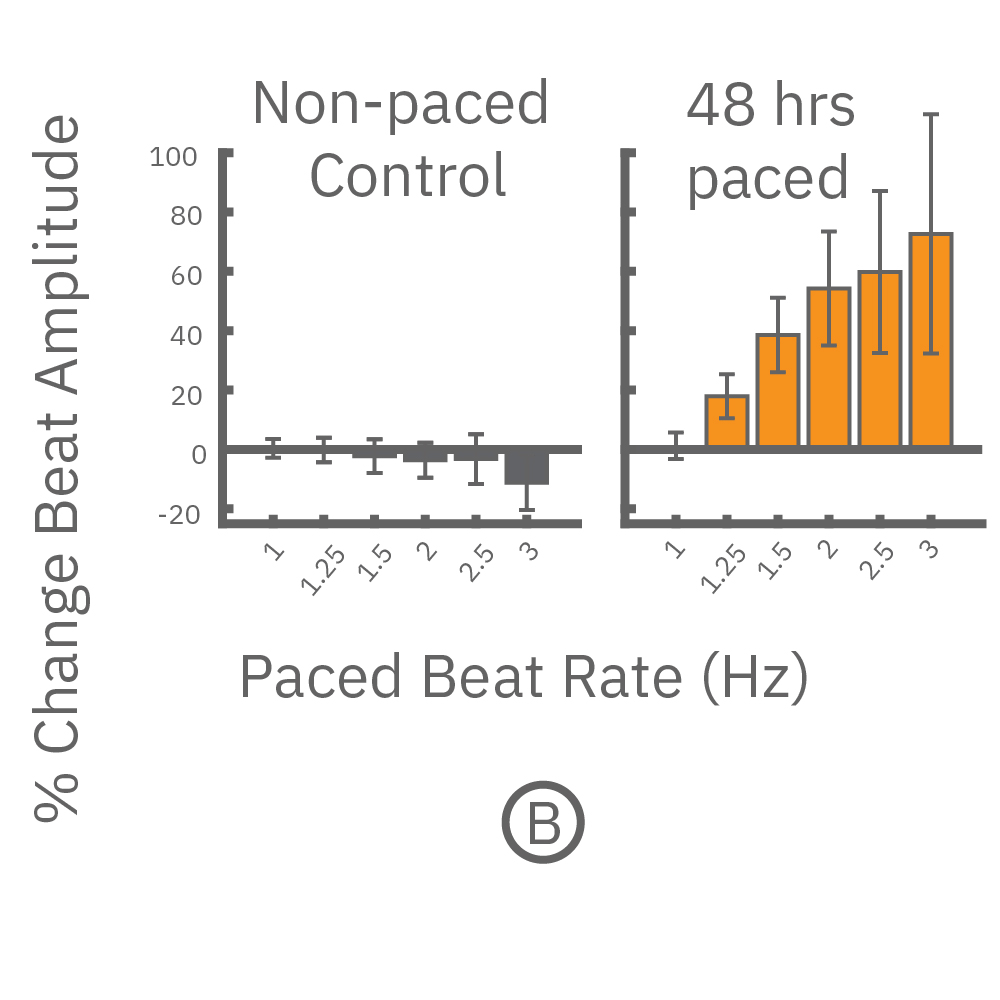
(B) 各刺激頻度毎の 拍動振幅値の変化を示す。連続ペーシングを印加した心筋細胞では、拍動振幅値の上昇が得られ(オレンジ色)、ペーシング無しの細胞では、わずかな減少が得られた(グレー色) 。
変力作用化合物の評価
ヒトiPS細胞由来心筋細胞の未成熟な表現型は、変力作用化合物への乏小な反応、特にβアドレナリン受容体アゴニストに対する陽性変力性応答の欠如や減少にて示されます。本事例では、Maestro MEAで2Hzのペーシングを48時間連続して行い、陽性変力化合物の評価を行いました。
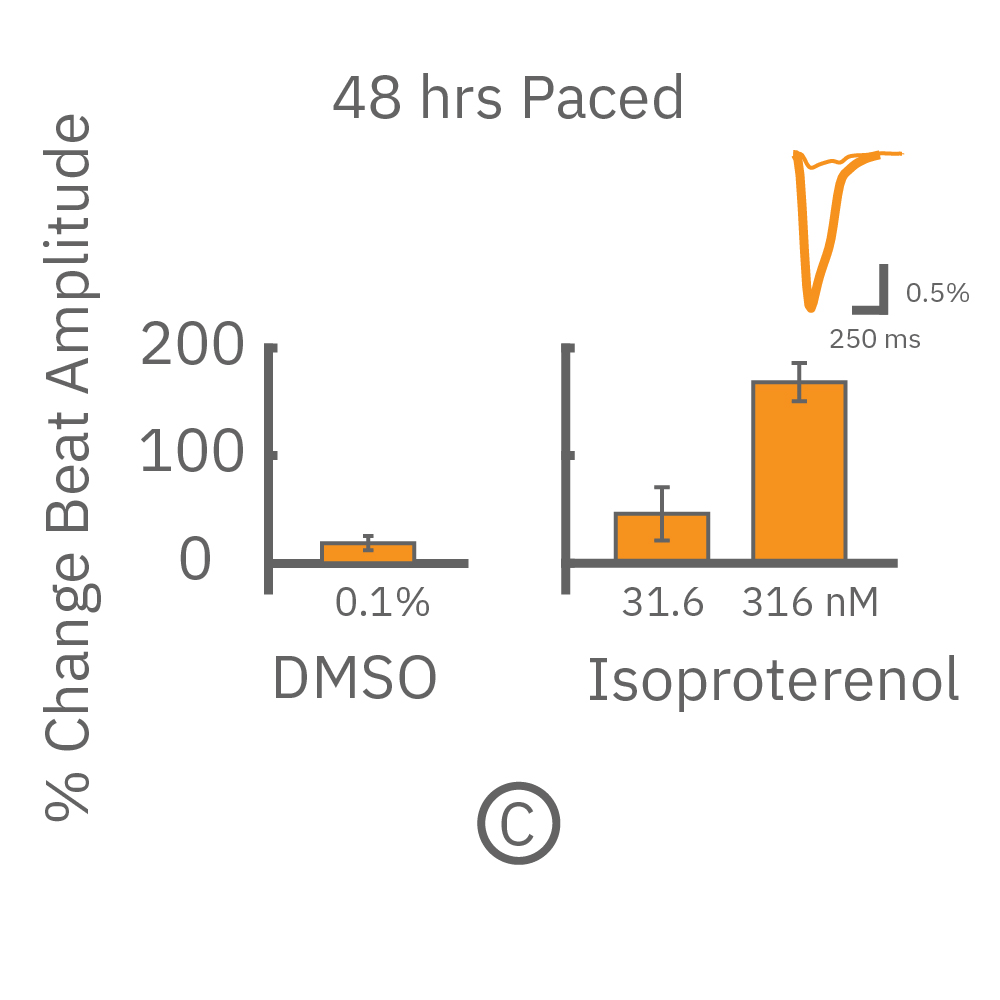
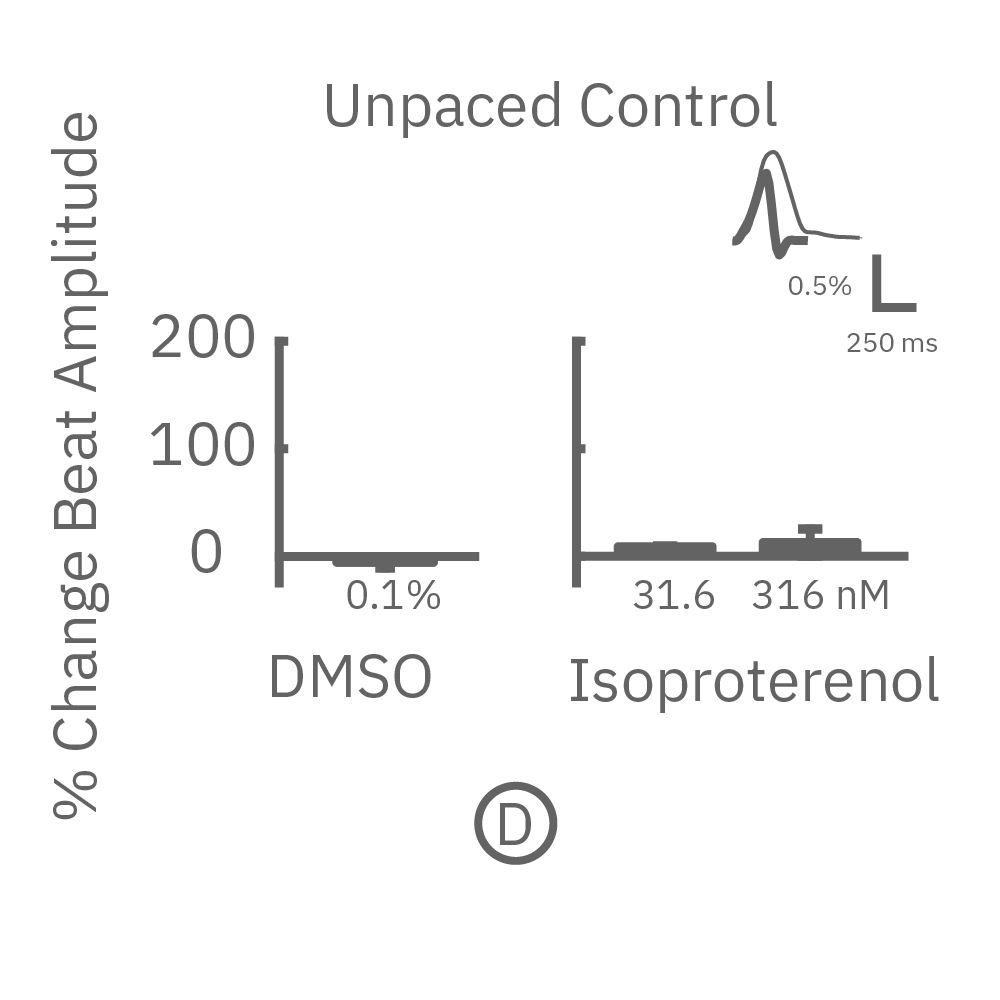
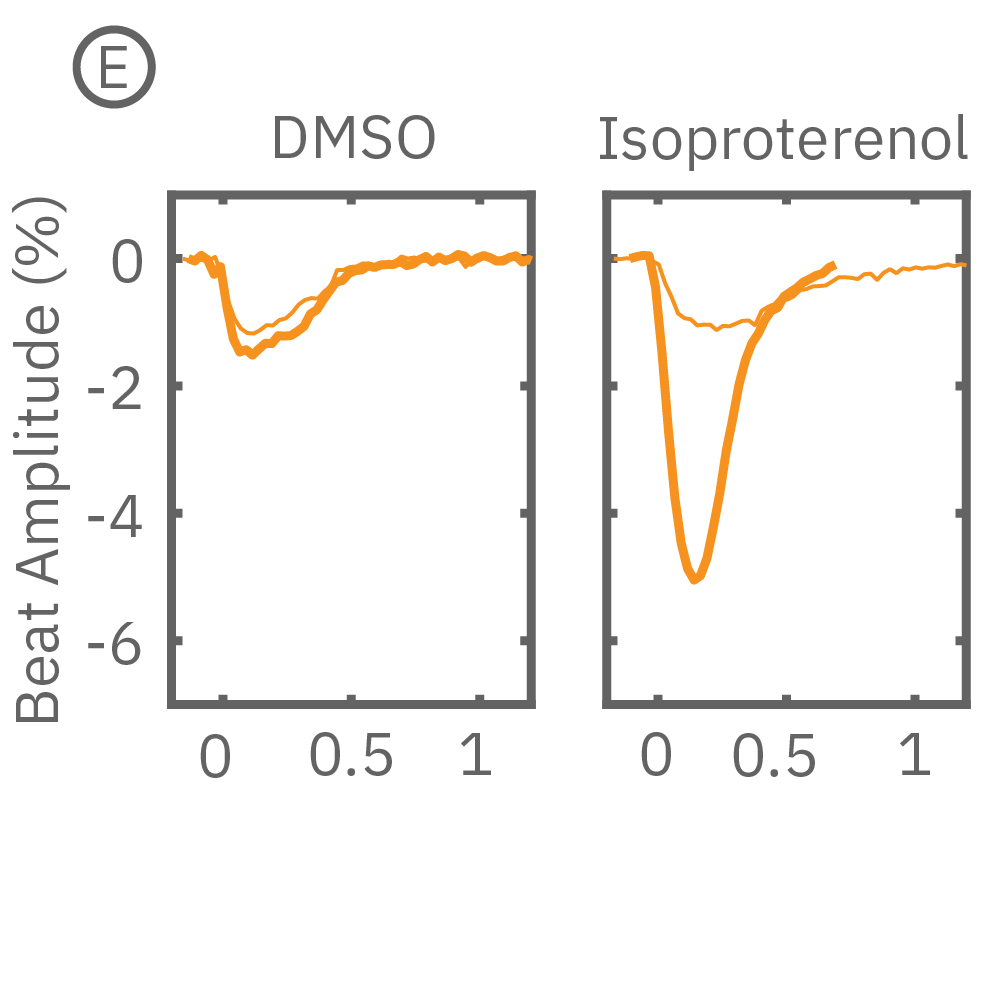
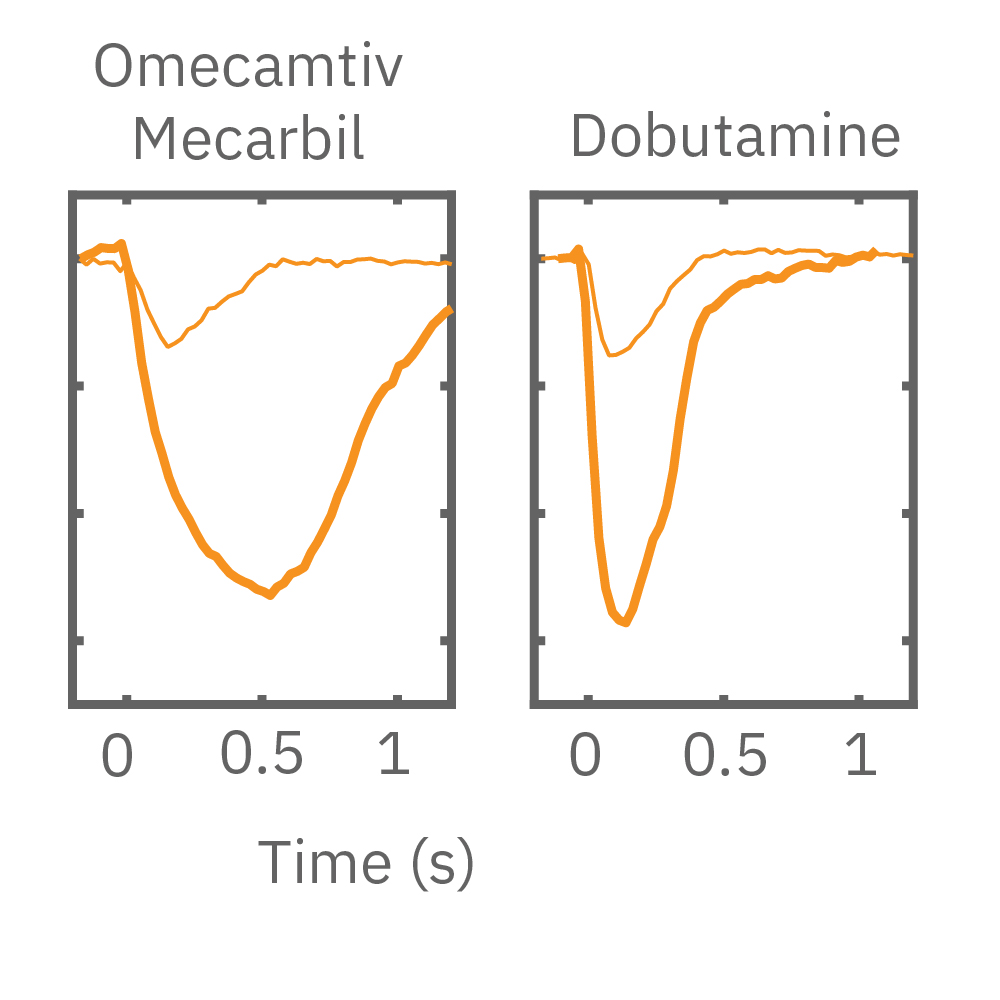
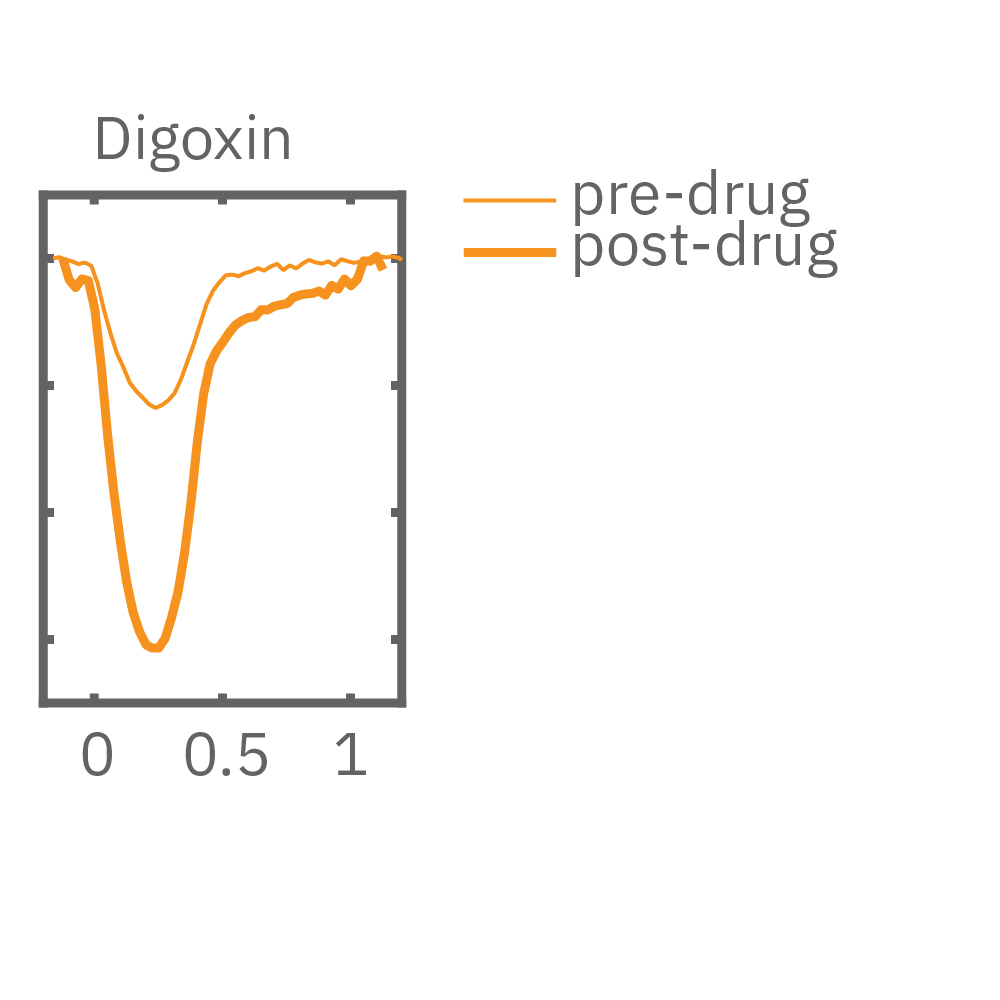
MEA プレート上に培養されたiCell CM² (富士フィルムCDI) に48時間連続して2Hzのペーシングを行った後、陽性変力作用薬(イソプレテレノールなど)を投与した。
(C) 48hペーシングを印加した心筋細胞の拍動振幅値の変化率を示す。イソプレテレノール (βアドレナリン受容体アゴニスト) の投与により、拍動振幅値の上昇が得られた。
(D) 48hペーシング非印加(コントロール)の心筋細胞の拍動振幅値の変化を示す。DMSO、イソプレテレノール共に顕著な変化は得られなかった。
(E) 48hペーシング印加した心筋細胞へのDMSO及び複数種類の陽性変力作用薬投与時の拍動波形の変化を示す。全ての変力作用薬に対して、拍動振幅値の上昇が得られた。
3D培養における収縮変化の検証
スフェロイド、オルガノイドなどの in vitro の3D 細胞モデルは、in vivo 組織の多層構造をより正確に再現することが可能であることから、疾患モデリング、発生生物学、安全性試験などにおいて、その有用性が提起されています。本事例では、MEAプレート上に播種された複数の3Dスフェロイドから Contractility (インピーダンス変化)を測定しました。
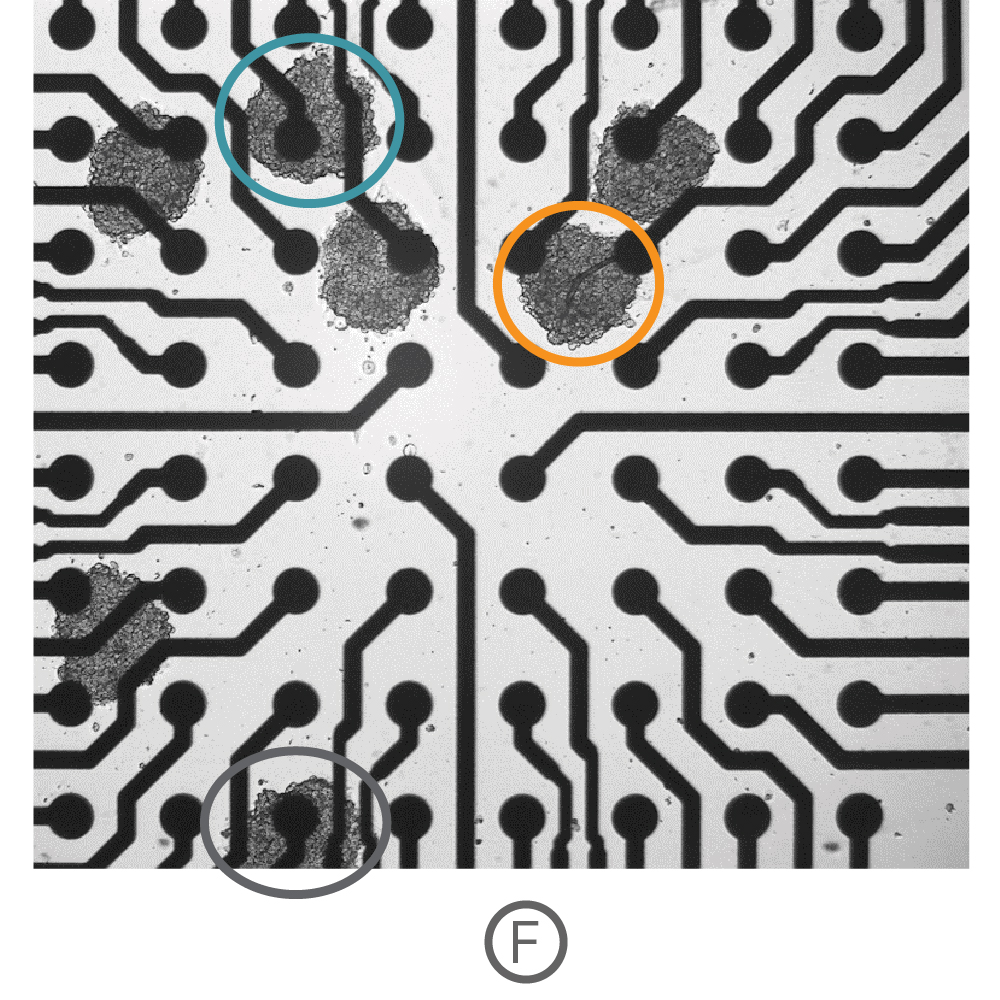
(F)複数の心筋細胞スフェロイド (Ncardia, Cor.4U) をCytoView 6well MEA プレート上に播種し、電極への接着後、測定を行った。
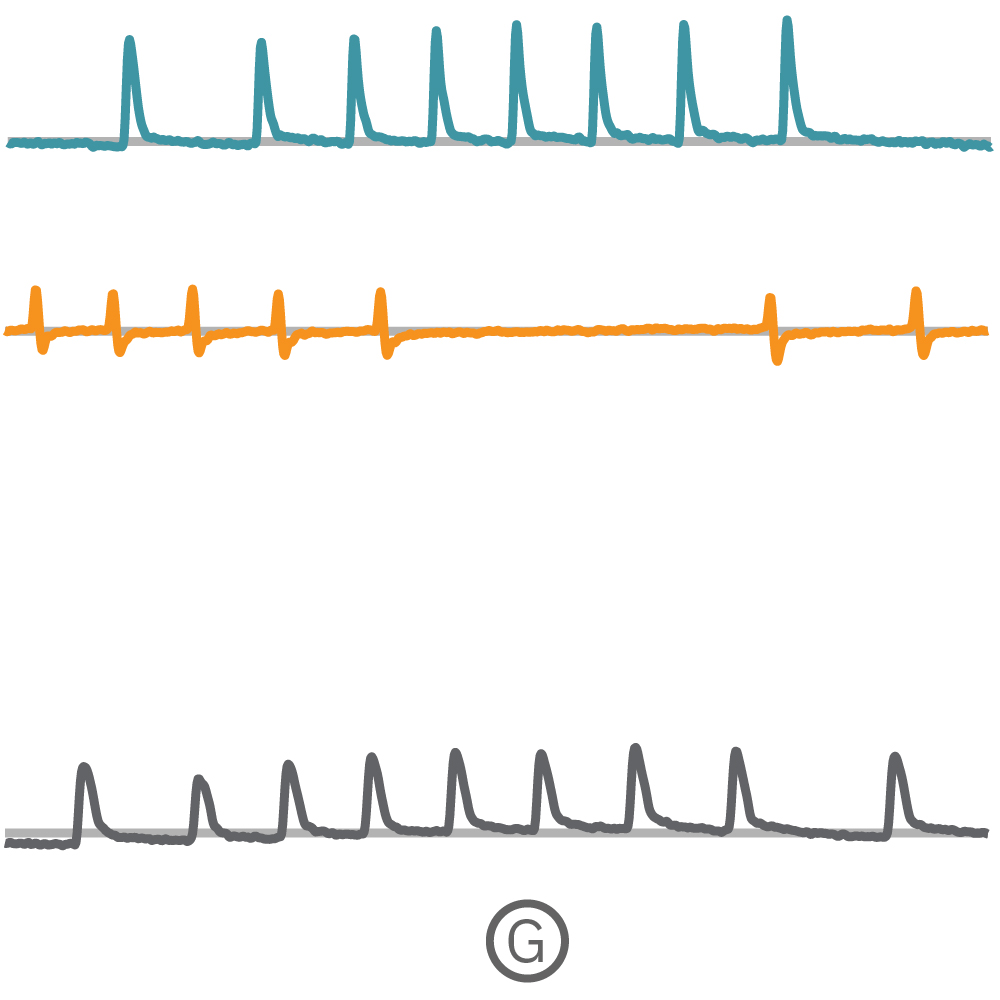
(G) Fで示された各電極から取得された Contractility波形を示す。
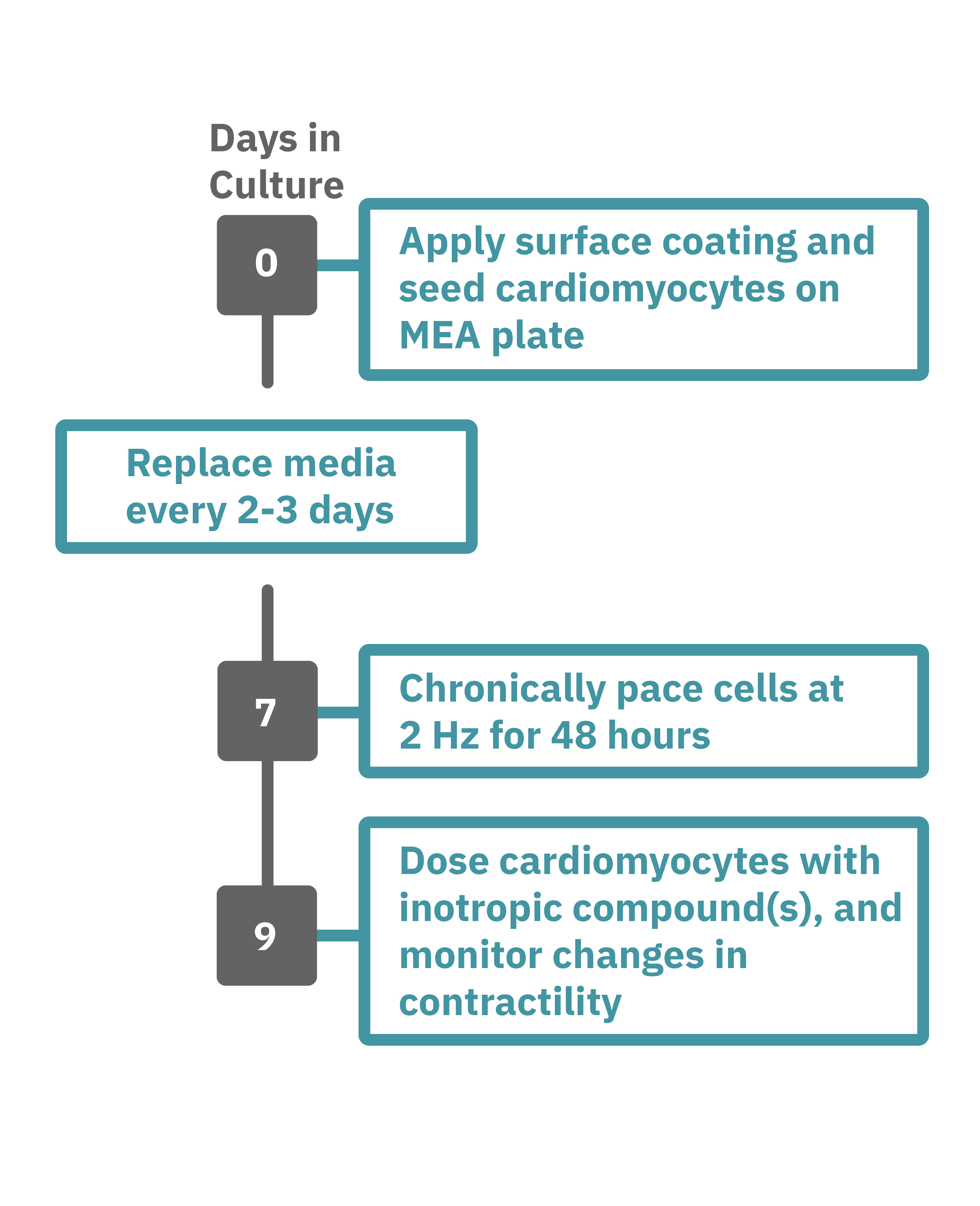
Maestro Pro/Edge による心筋細胞弛緩収縮評価は非常に簡単です。
コーティングされたMEAプレート上に直接心筋細胞を播種します(Day 0)。2-3日毎に培地交換をしながら、細胞を培養します。
一定の成熟が得られた後 (例: Day 7+)、プレートを Maestro に搭載し2Hzのペーシングを48時間に渡り連続して印加します。48時間後、ペーシングを停止し、変力作用薬などを投与してデータ測定を行います。データは付属のソフトで解析可能です。

Maestro MEA による心筋細胞弛緩収縮評価:特徴
-
48時間ペーシングによるiPS細胞由来心筋細胞の変力作用評価 - 電気的な刺激は、iPS細胞由来心筋細胞を成熟化させる⼿段の1つとして有効性は⽰されていますが、現在の手法では、成熟の指標の1つとされる Force-frequency relationship (FFR) を得るのに数週間を要します。Maestro MEAによるペーシングでは、48時間の連続刺激でFFRが得られ、変力作用化合物の評価が可能です。
-
1システムで4種類のアッセイ - Maestro Pro/Edgeでは、1枚のプレートで次の4種類のアッセイが行えます。[1] 細胞外電位応答 [2] シグナル伝播 [3] Contractility (弛緩収縮評価) [4] LEAP (活動電位形態シグナル)
-
同一プレート上で培養から測定まで - MEAプレート上で直接細胞を培養し、測定します。環境要因による変化を最小限にとどめながら、細胞への負担が少ない状態で、細胞の変化を検出することができます。
-
ラベルフリー・リアルタイムで細胞の電気的な活動を測定 - プレート上の電極を用いて心筋細胞の活動電位を測定します。ラベルフリー、リアルタイムな測定は、試薬など2次的要因によるゆがみがなく、細胞の変化をより正確にとらえます。また、数日から数週間に渡る慢性評価にも最適です。
-
安定した環境下での実験 - 温度・CO₂ 濃度は、装置搭載のコントローラで自動制御されます。また、Maestro は外来ノイズ・振動に影響されにくい設計になっています。安定した環境で毎日安心して実験に望めます。
-
簡単操作 - 電気生理未経験の方でも簡単に実験が行えます。MEAプレート上に細胞を培養し、装置に搭載するだけで、心筋細胞の電気的な活動の測定が可能です。付属のソフトウエアパッケージを用いて、数クリックで複数の指標に基づいた解析結果が得られ、結果の作表まで行うことができます。

Cardiac MEA
Show Full DetailsWhat is Cardiac MEA
Cardiac MEA (microelectrode array) technology is a label-free method for recording electrical activity in heart cells. Tiny electrodes embedded in the culture surface detect electrical signals from cardiomyocytes, allowing continuous, noninvasive monitoring of cardiac function in real time. This technology provides insights into cardiomyocyte function, rhythm, and drug response in 2D and 3D cell models
Why Cardiac MEA matters
Traditional cardiac assays rely on indirect, low resolution, or endpoint measurements that fail to capture electrical function, rhythm changes, and time-dependent drug effects. Cardiac MEA technology addresses this gap by providing direct, physiologically relevant measurements of cardiac electrophysiology, supporting safer drug development, improved cardiotoxicity screening, and more predictive disease models.
Contractility
Show Full DetailsWhat is Contractility?
Every beat of the heart is characterized by a contraction of the heart that pumps the blood out to the body. When cardiomyocytes are cultured on top of electrodes, they form a spontaneously beating syncytium. With each beat, the cells contract and relax, changing their shape and coverage over the electrodes. These changes can be measured as a change in impedance, or contractility.
Why Contractility Matters
Contractility is often used to characterize the mechanical properties of induced pluripotent stem cell-derived cardiomyocytes and to detect the effects of compounds on cardiac contractile function (e.g., inotropes). Measures such as beat amplitude, beat period, and excitation-contraction delay reveal changes in contractile function due to cardiomyocyte maturation or compound addition.
Optogenetics
Show Full DetailsWhat is Optogenetics?
Optogenetics is a technique that involves the use of light to control cell function. Cells are first genetically modified to express light-sensitive ion channels, called opsins. Then, light can be used to activate the opsin. The most well-known opsins are light-gated ion channels that can control the excitability of the cell membrane.
Why Optogenetics Matters
By delivering precise, time-locked stimulation, optogenetics enables researchers to interrogate circuit behavior, model disease-related dysfunction, and evaluate therapeutic interventions with high temporal and spatial resolution.