心毒性は、自然及び人工の物質によって引き起こされる心臓の機能障害や損傷を意味します。創薬において、臨床試験前の段階で治療化合物の心毒性を予見することは極めて重要です。
Maestro MEA は、in vitroでの心毒性評価に広く使用されています。プレート上に埋め込まれた複数の平面微小電極を用いて心筋細胞の電気的な活動を測定します。複数電極による測定は、心筋細胞のシグナル伝播の評価にも最適です。また、同一電極を用いての弛緩収縮の評価も可能で、1度のアッセイで多くの情報が得られます。
心筋細胞を用いた安全性試験
The Comprehensive in vitro Proarrhythmia Assay (CiPA) は新薬の催不整脈リスク評価系の改善を目的としてその活動が行われています。CiPA パイロットスタディでは6施設がMaestroを使用し、2種類のiPS細胞由来心筋細胞を用いて8種類の既知化合物の評価を行いました。Maestroによる薬剤応答の変化の検出は正確で、また、施設間及び異なる細胞種において高い再現性が得られました。
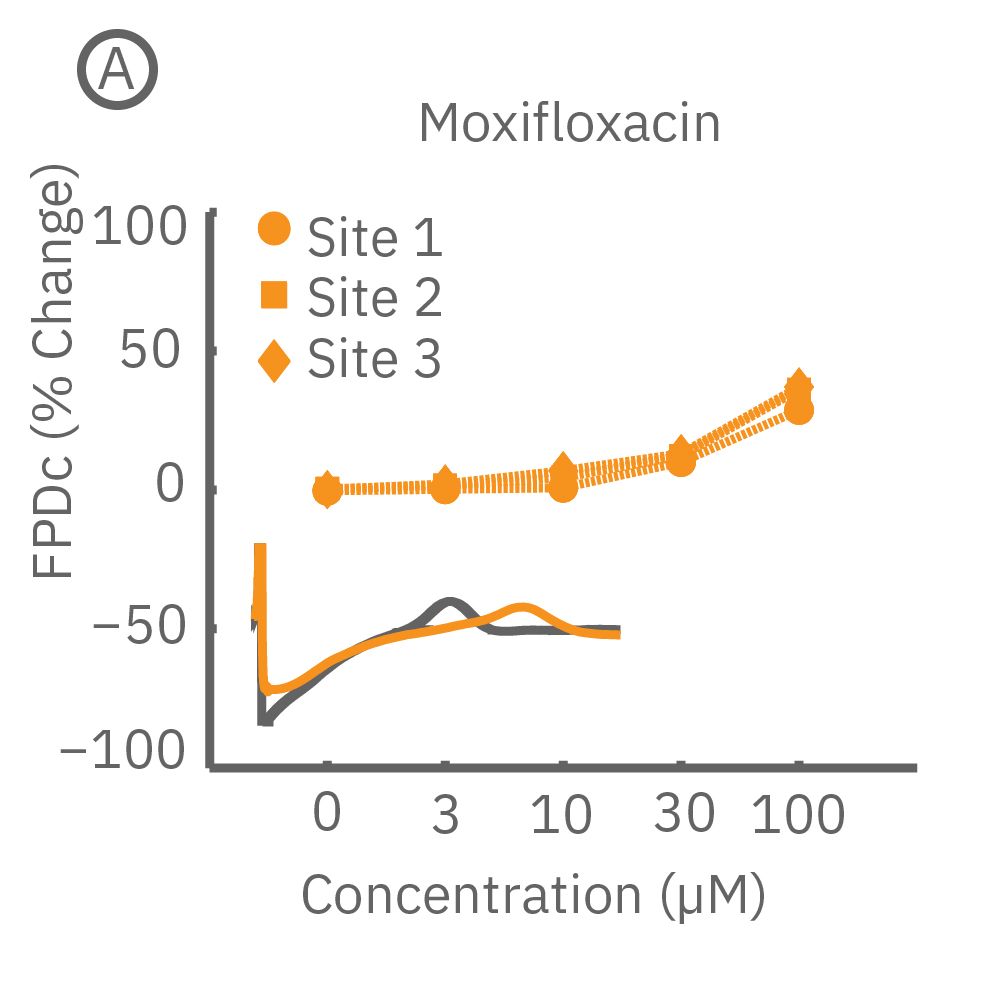
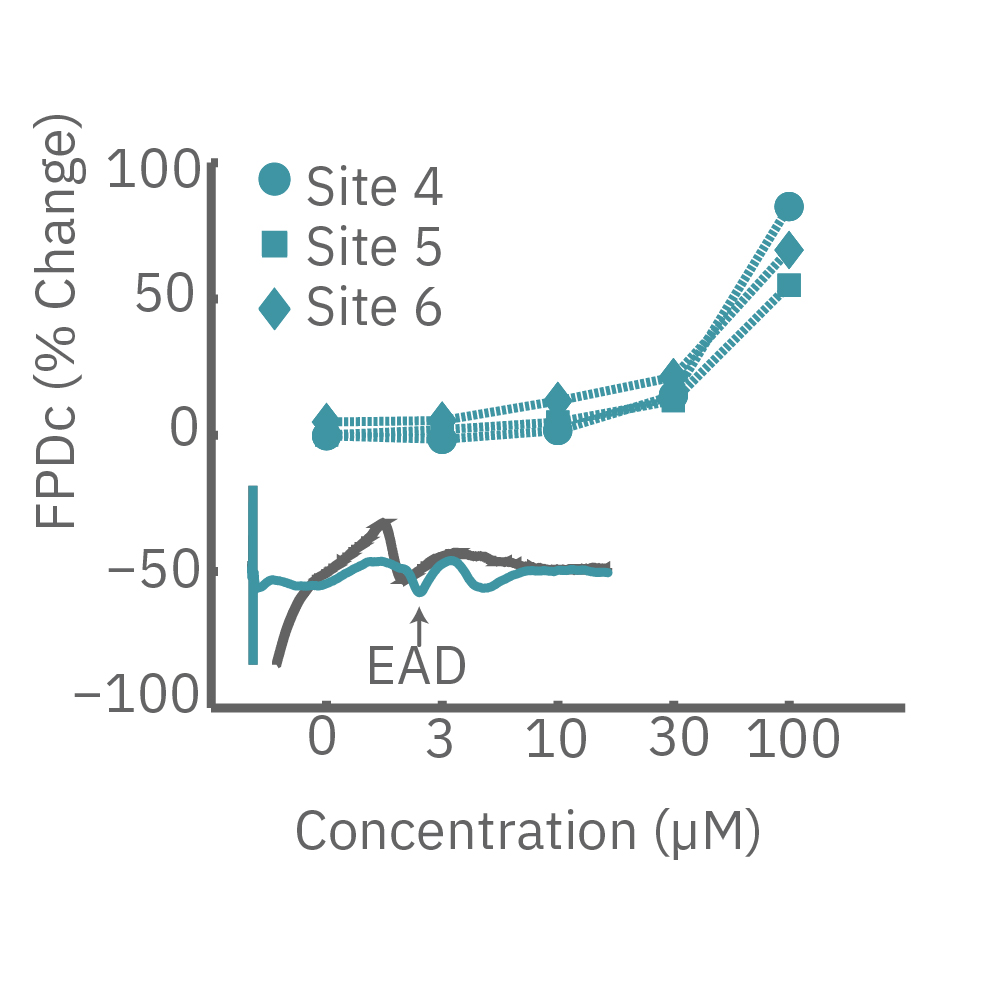
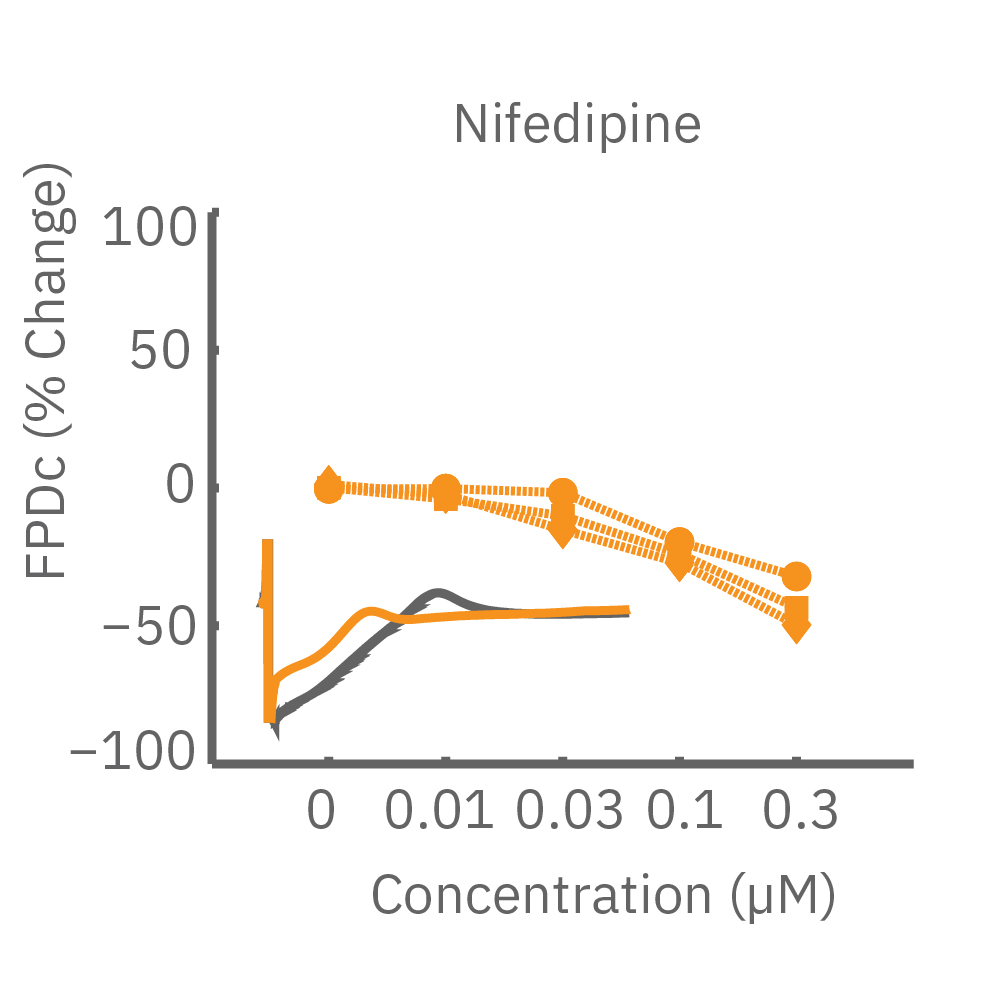
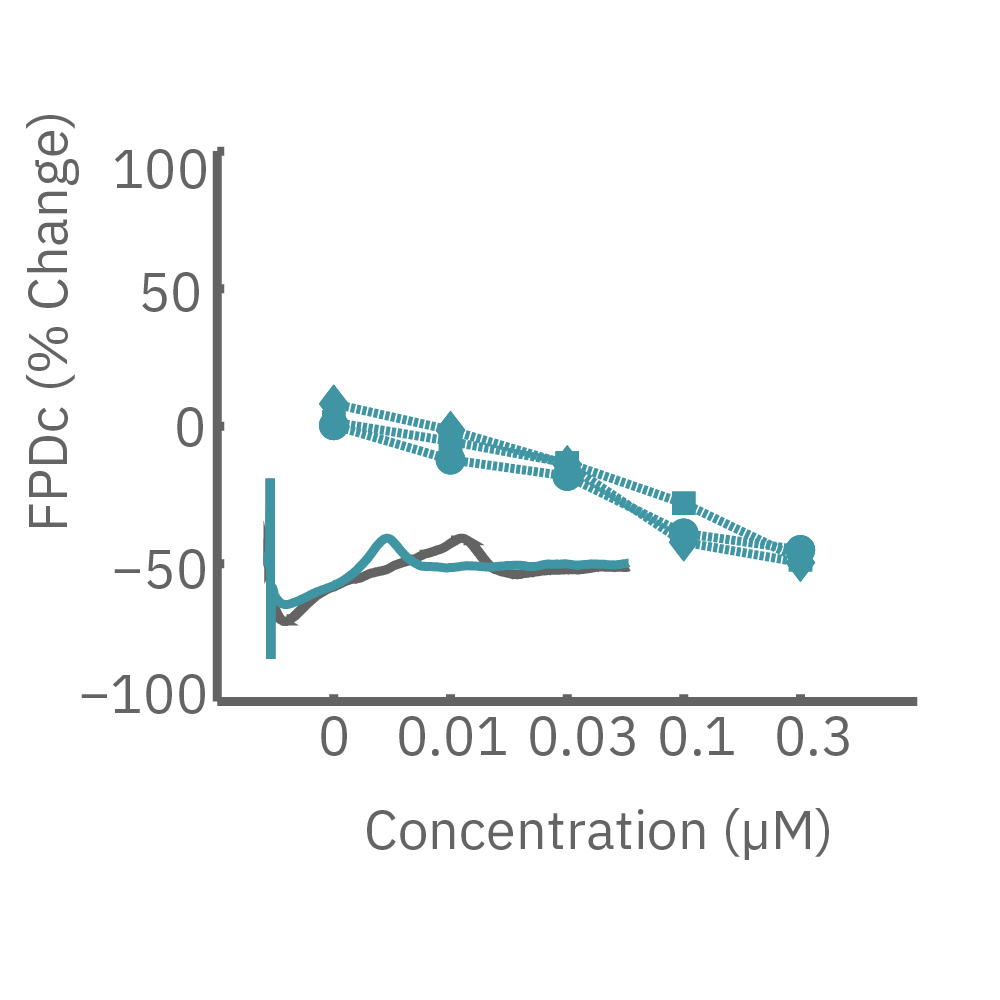
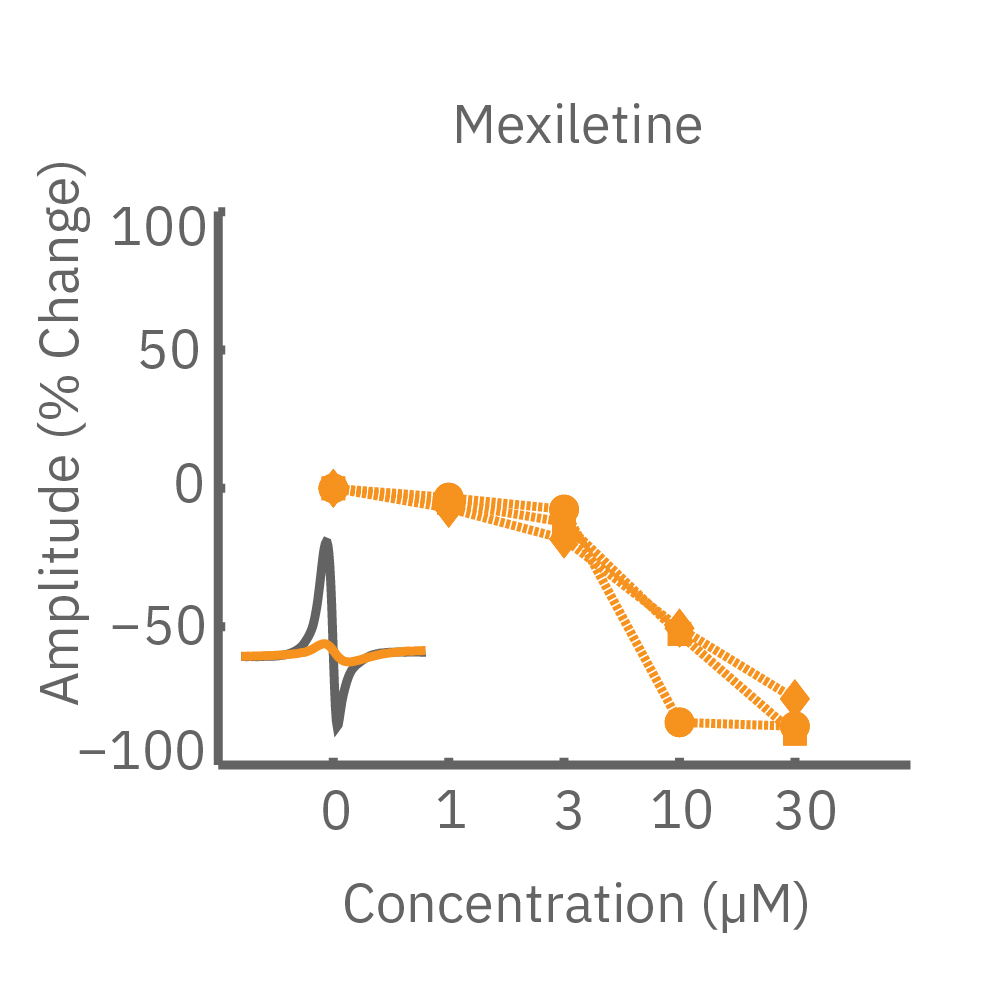
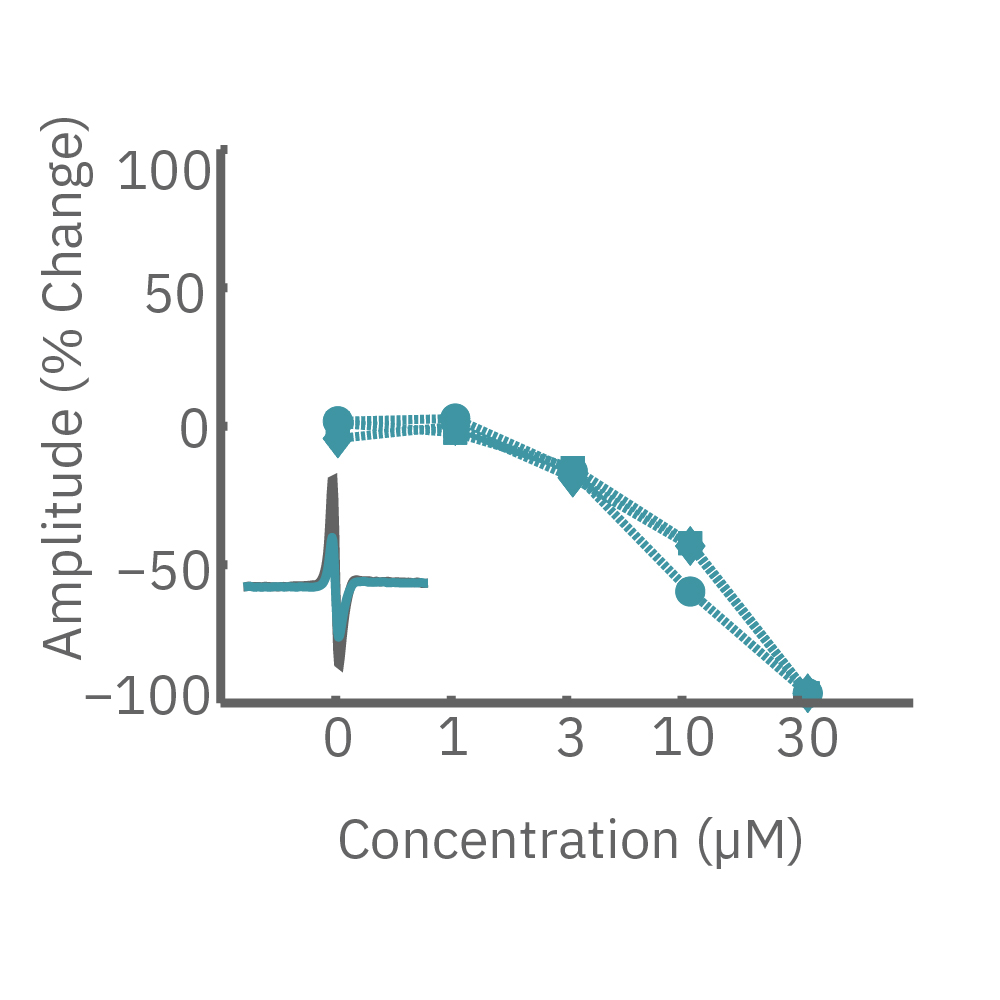
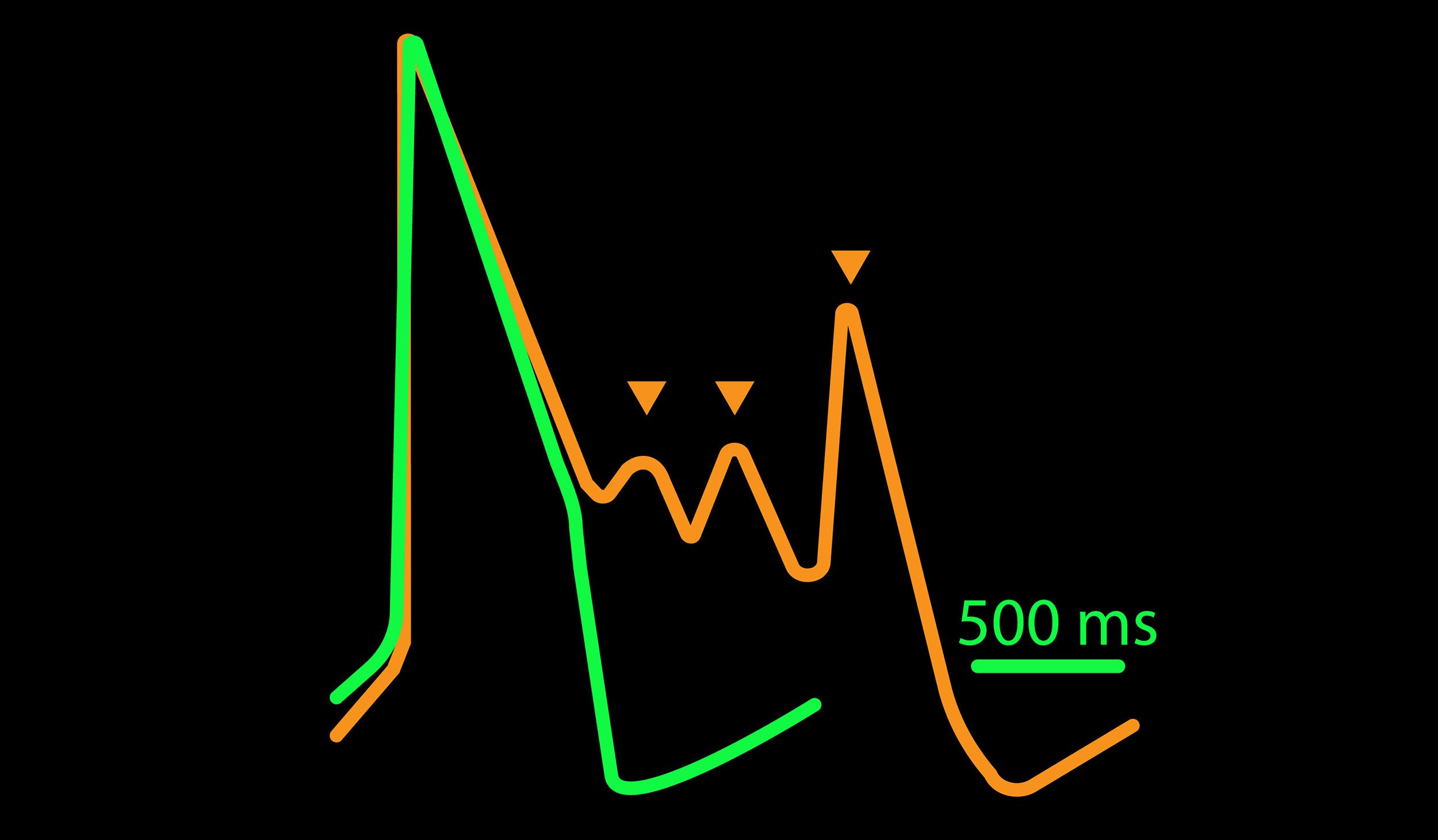
CiPA パイロットスタディにおける Maestro 測定結果の一部。(A) は6施設よる、2種類のiPS細胞由来心筋細胞 (Cor4 U/オレンジ (Axiogenesis社)・ iCell/水色 (CDI社)) を用いた3種類の化合物評価の結果を示す。
Moxifloxacin (hERG K+ 阻害剤) の投与でFPD (Field Potential Duration) の延長(左)、Nifedipine (Ca2+ 阻害剤) の投与でFPDの縮小 (中央) が得られ、Mexiletine (Na+阻害剤) の投与ではスパイク振幅値の減少(右) が得られた。また、同様の結果が複数施設、2種類の心筋細胞に渡り再現された。
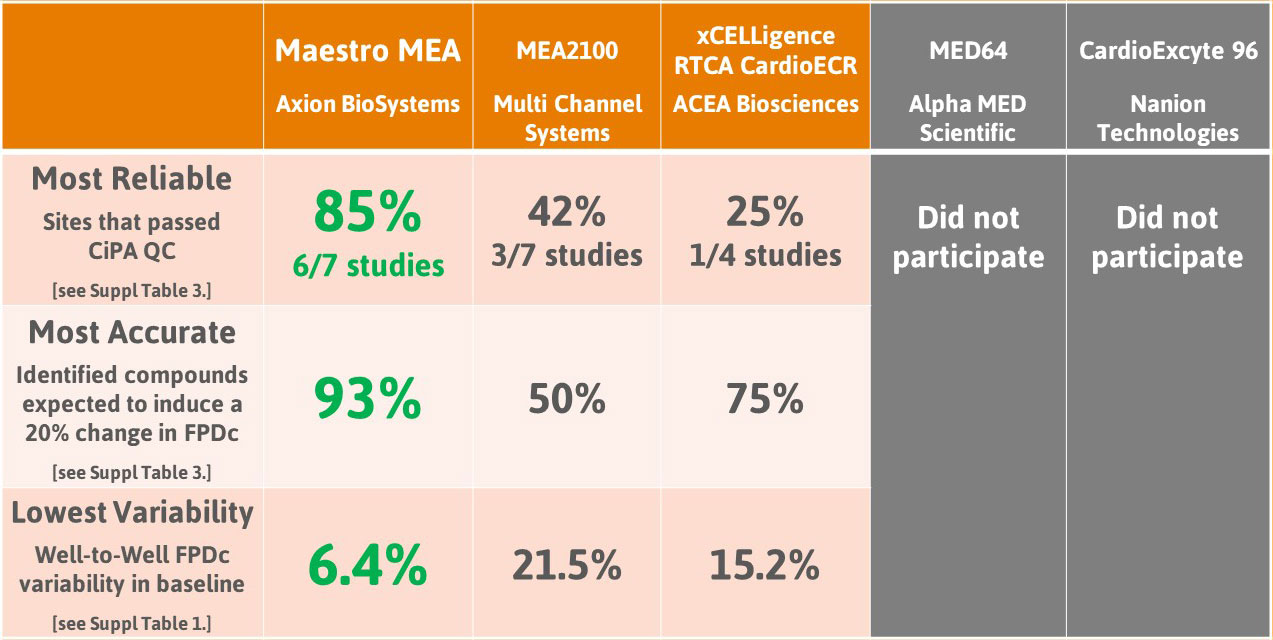
複数施設による未知化合物の評価を行ったCiPA パイロットスタディでは、iPS細胞由来心筋細胞が、心臓に影響を及ぼす化合物の検出に有用であることが実証されました。Axion BioSystems は本スタディで重要な役割を担ったことを誇りに思い、また参加された Maestro ユーザー様に感謝いたします。
Download CiPA Pilot Study Paper
パイロットスタディに続いて実施された CiPA Myocyte Phase II validation study では、先の Phase I スタディの領域を拡大し、低・中・高の TdP (torsades de pointes) リスクを有する28 の薬剤を11施設にて評価しました。本 スタディでは、iPS細胞由来心筋細胞が、薬剤による不整脈反応の検出において有用であることが実証されました。Axion BioSystems は本スタディにおいてもリーディング的な役割を担ったことを誇りに思い、参加された Maestro ユーザー様に深く感謝いたします。
Download CiPA Phase II Study Paper
LEAPによるヒトiPS細胞由来心筋細胞を用いた薬理試験
LEAP (Local extracellular action potential) は、Maestro Pro/Edge の特有機能です。プレート底面の平面電極を用いて、Action Potential 形態シグナルを測定します。EAD (early after depolarization) などがよりクリアに検出されます。薬剤投与などにおいては、パッチクランプのシグナル変化と同様の変化を示します。
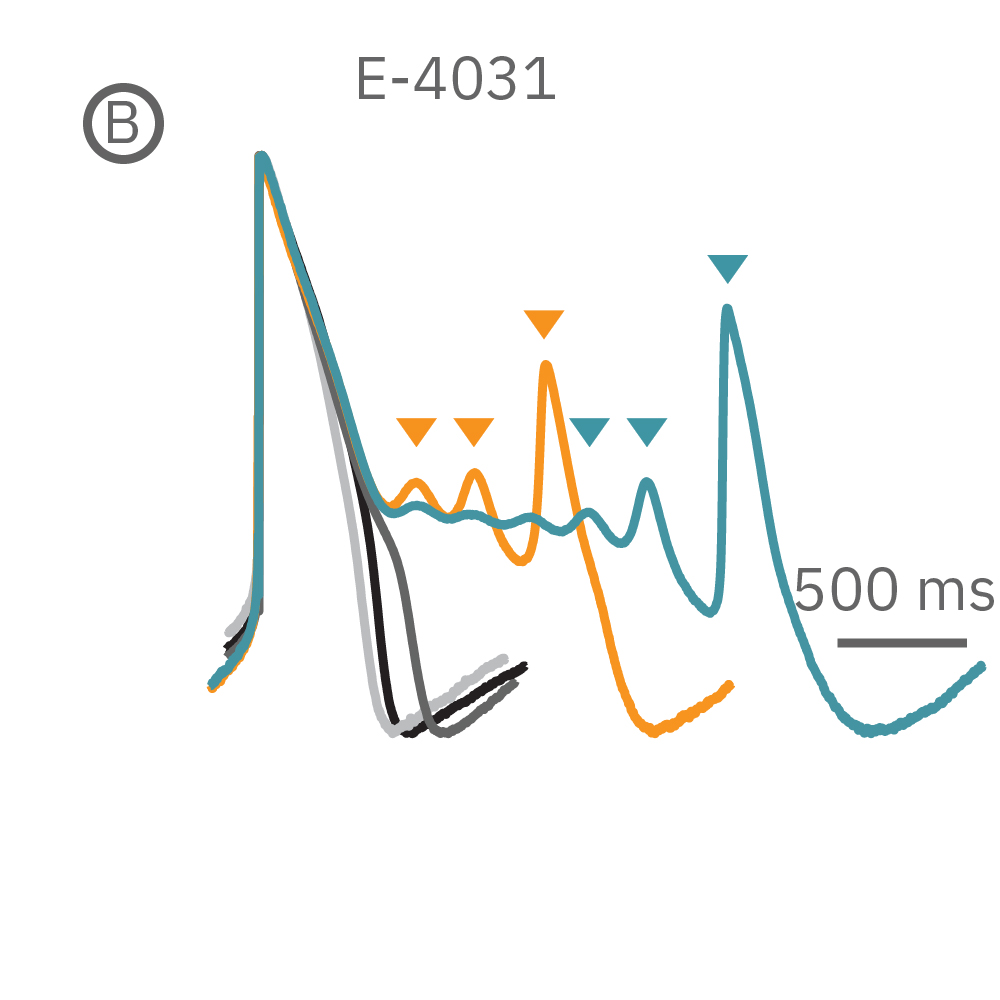
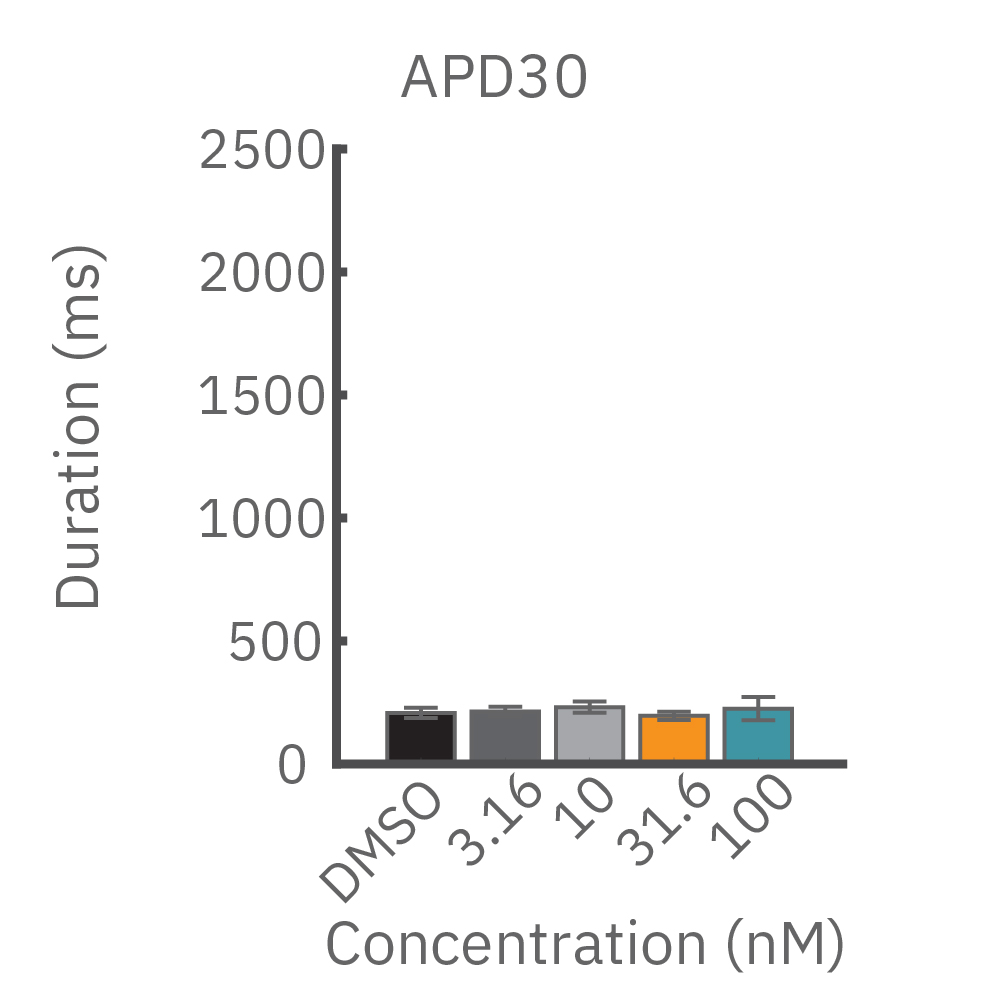
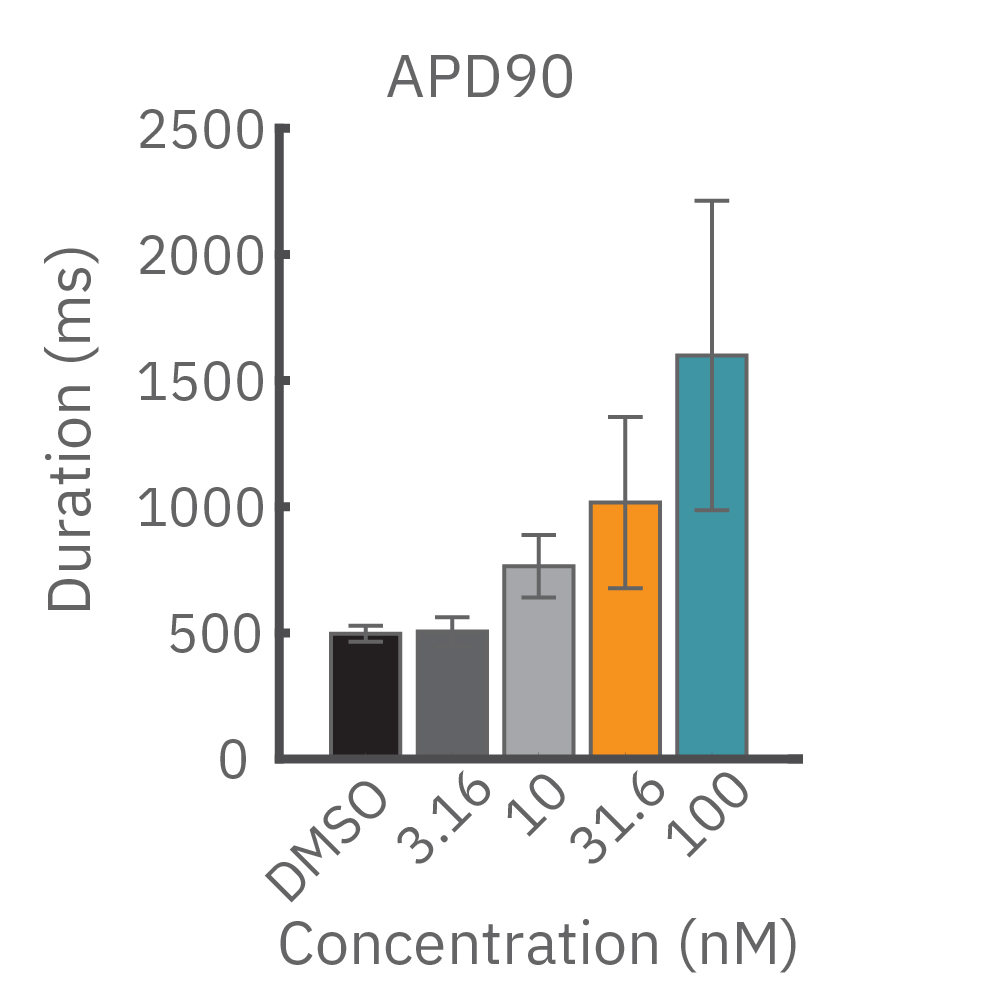
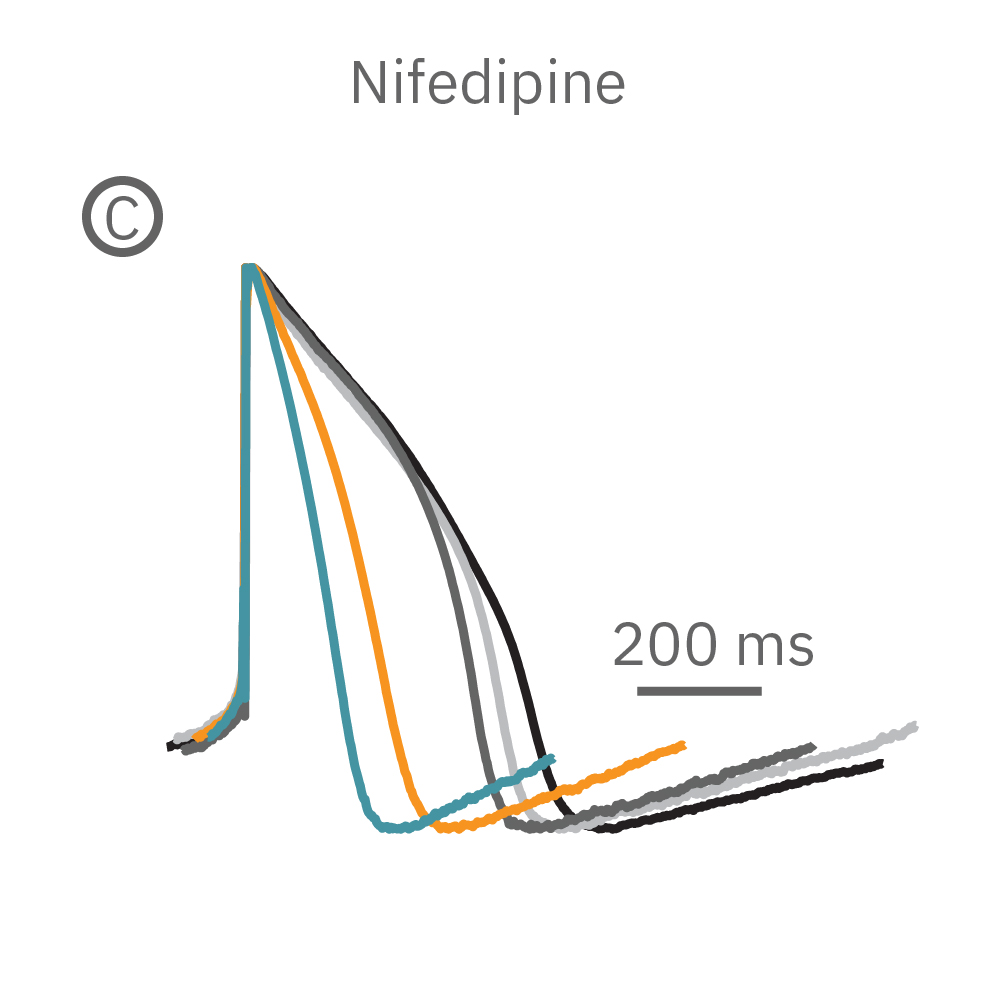
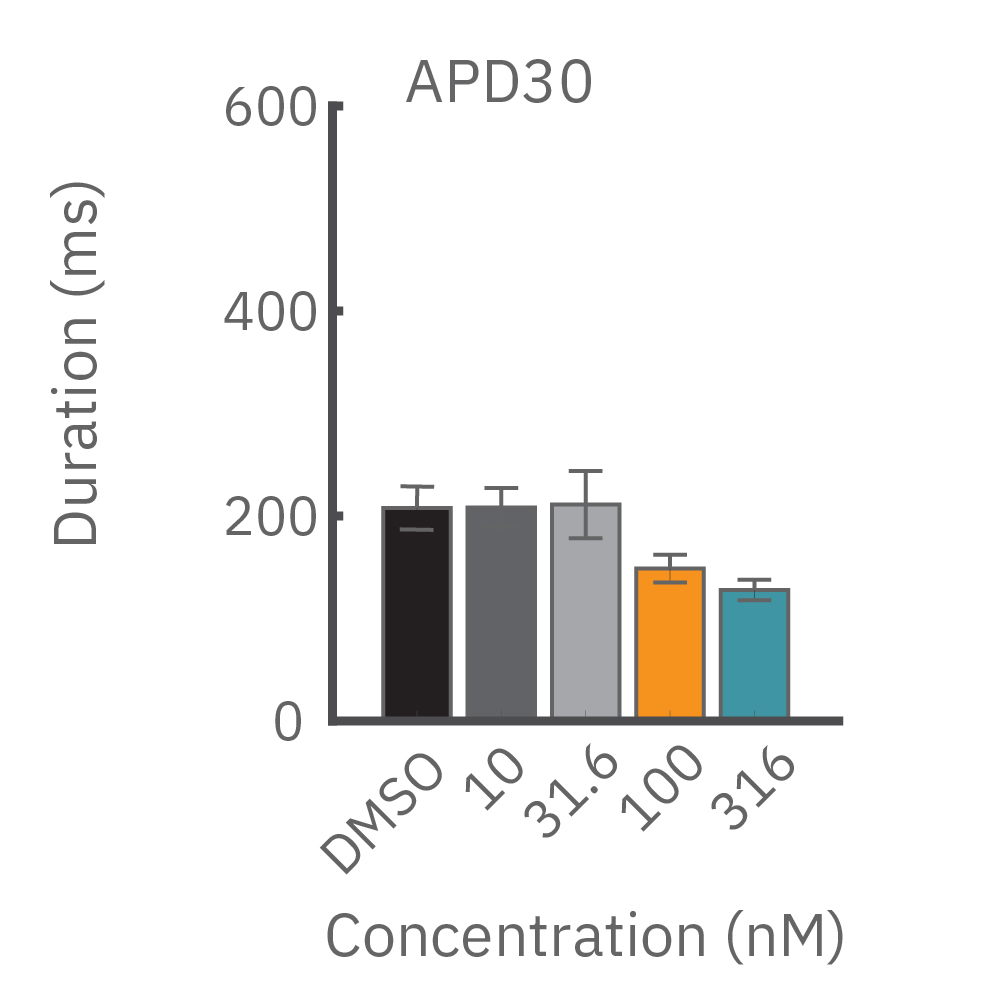
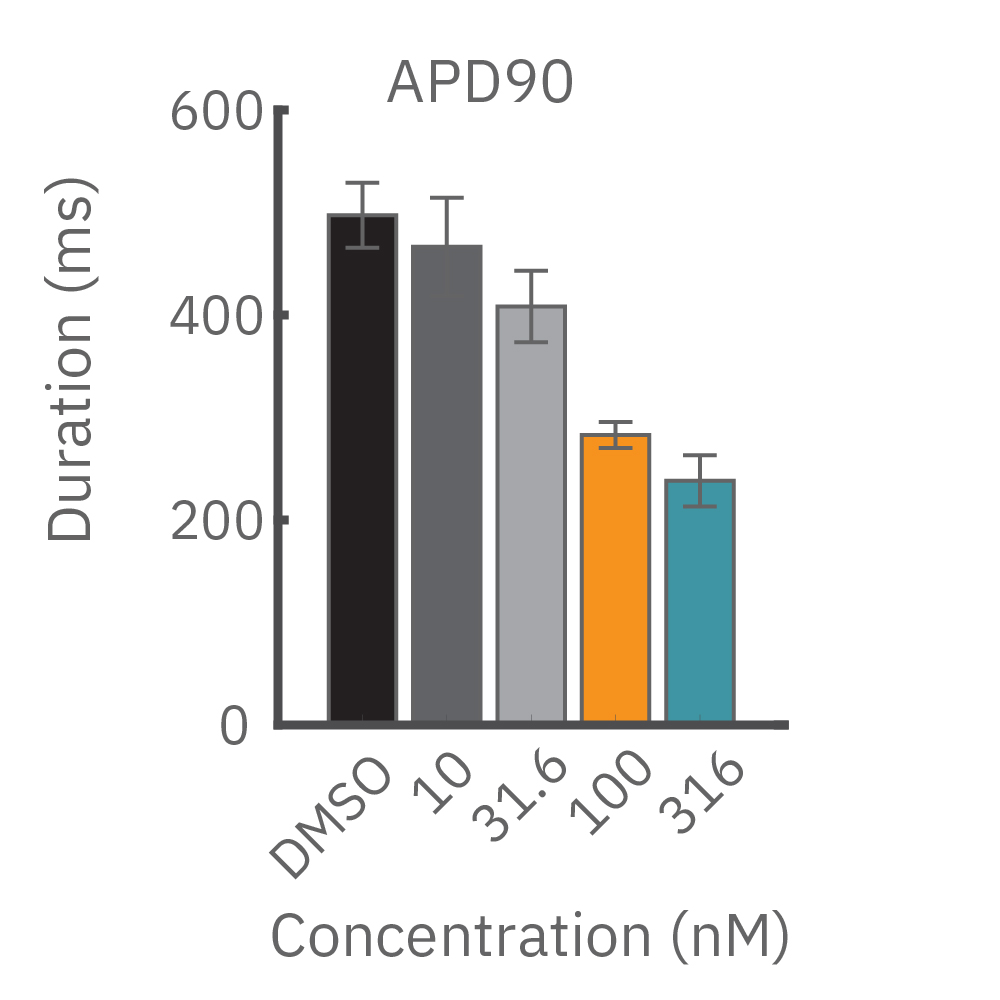
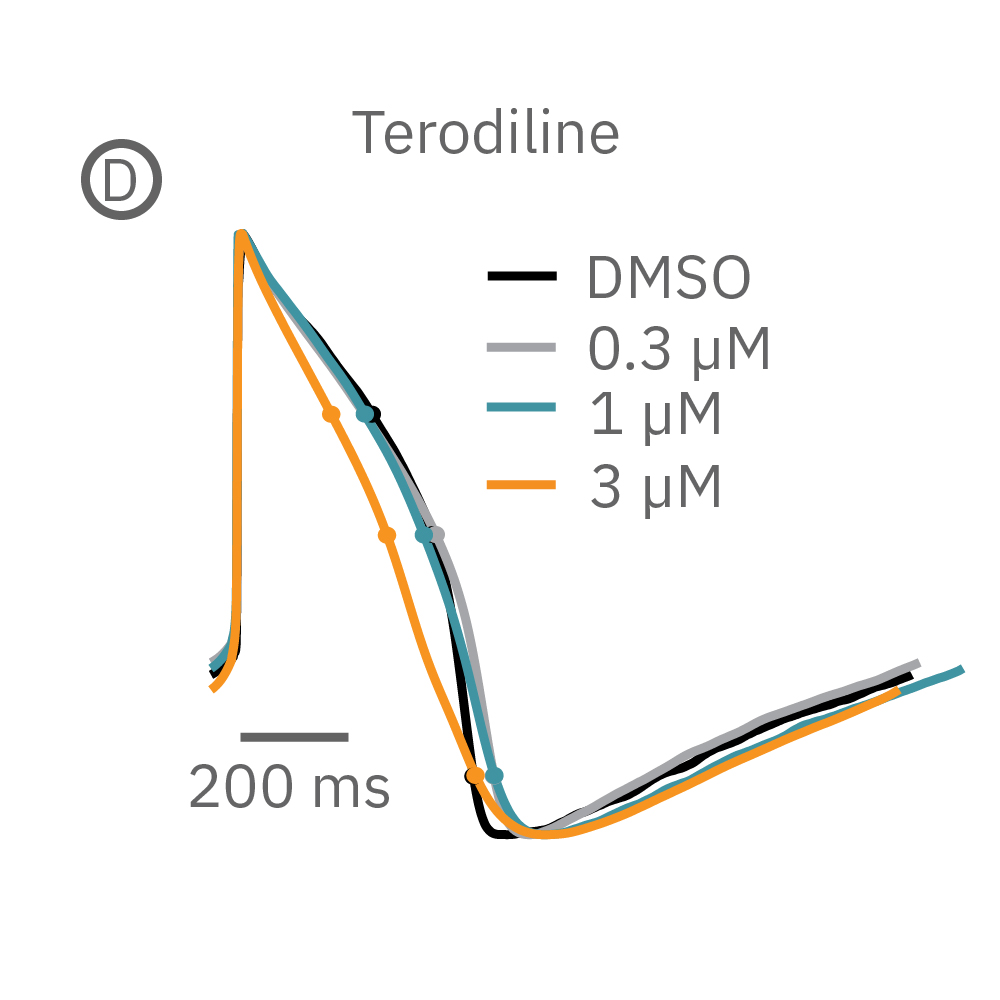
(B) E4031 投与による LEAP シグナルの変化。再分極フェーズの遅延と、EADを示唆する非規則性が得られた。
(C) Nifedipine 投与による変化。LEAP シグナルの持続時間は濃度依存的にに縮小した。
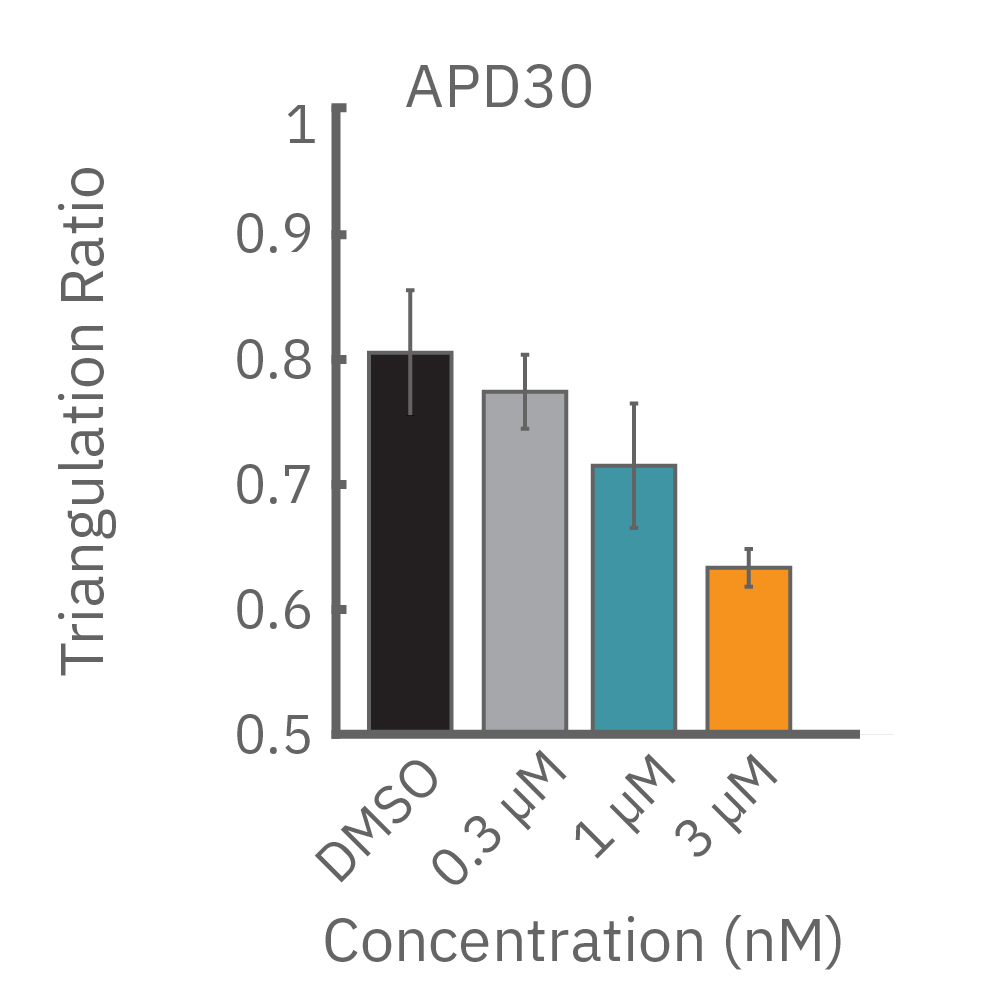
(D) Terodiline 投与による変化。高濃度化において三角形化 (triangulation) が見られ、パッチクランプデータとの相関が得られた。
化合物による伝播パターン及び伝播速度の変化の検証
iPS細胞由来心筋細胞合胞体においては、拍動は培養内の1点で発生し (pacer region)、培養内で波のように伝播します。薬剤や心疾患は、細胞の興奮性や細胞間のギャップ結合を改変させ、この伝播に影響を及ぼすことがあります。Maestro MEAによる複数電極からの電位応答測定は、これらの検証に最適です。
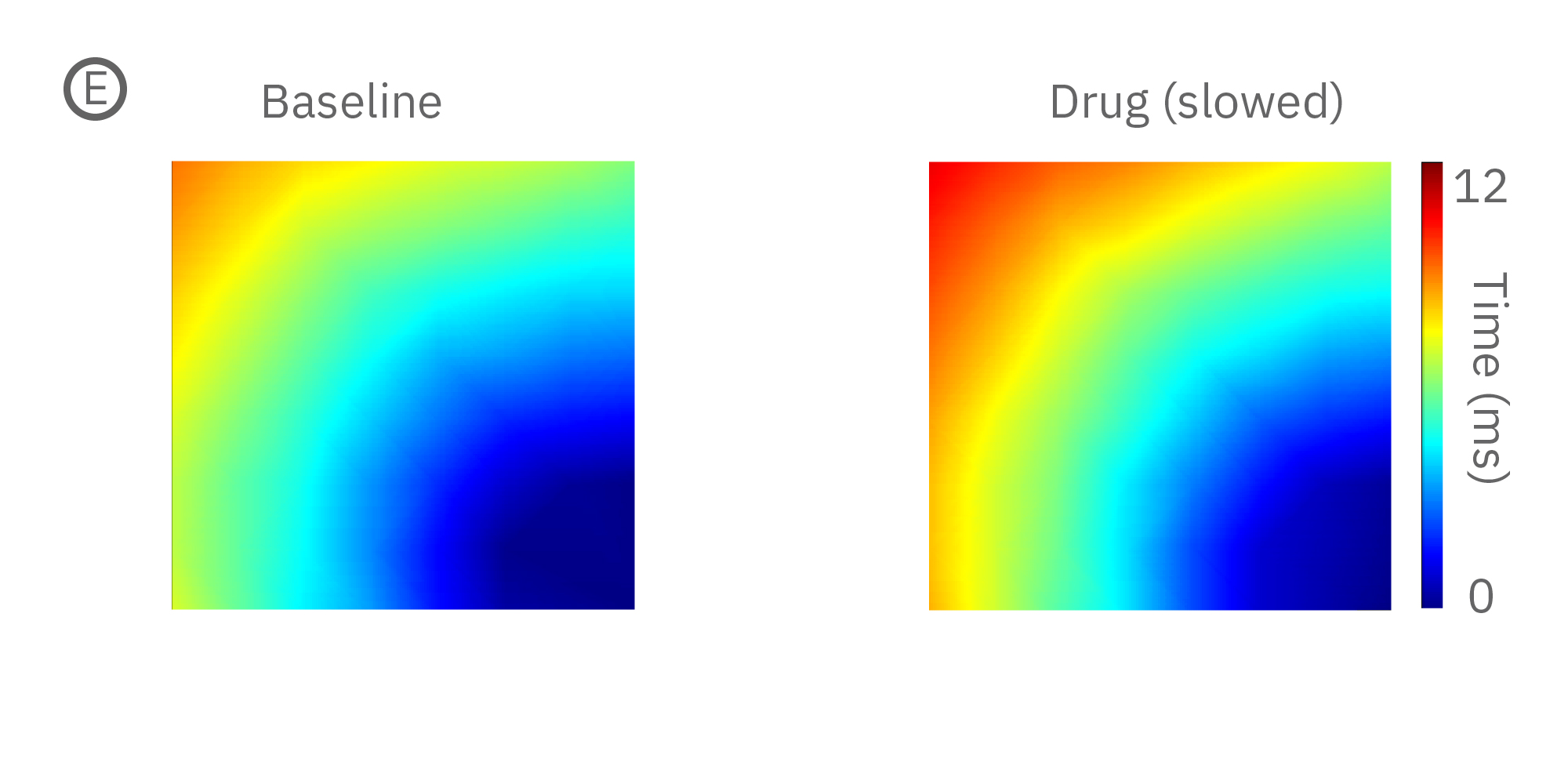
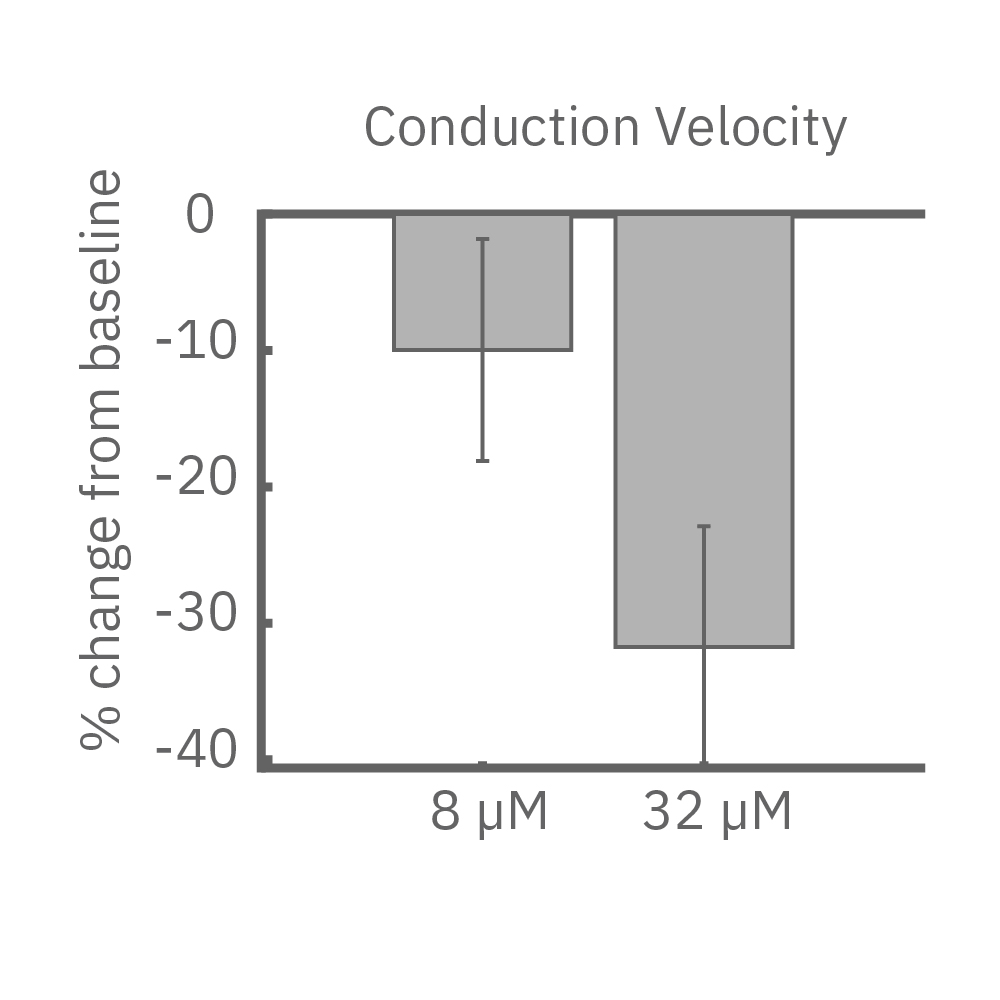
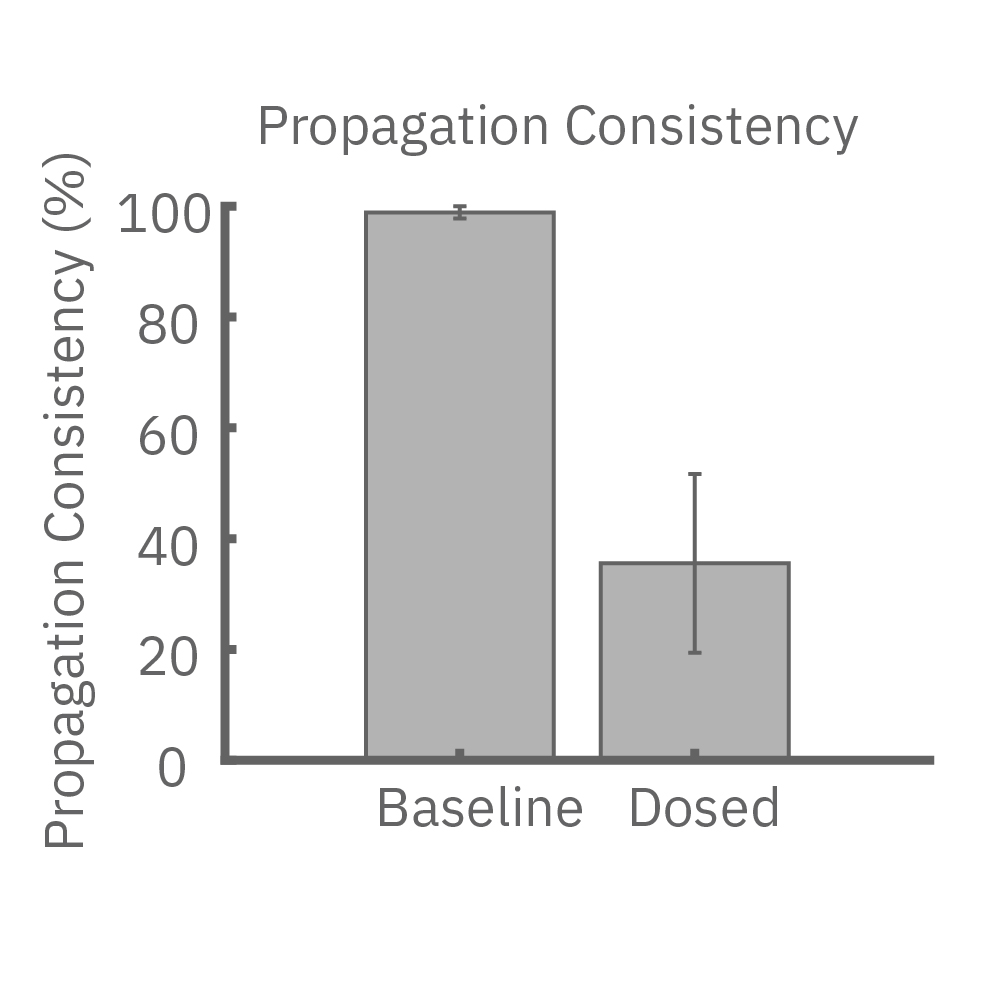
(E) iPS細胞由来心筋細胞に、Na+チャネルブロッカーを投与した際の伝播マップ(左>中央)と伝播速度の変化 (右)。濃度依存的な伝搬速度の減少が見られた。
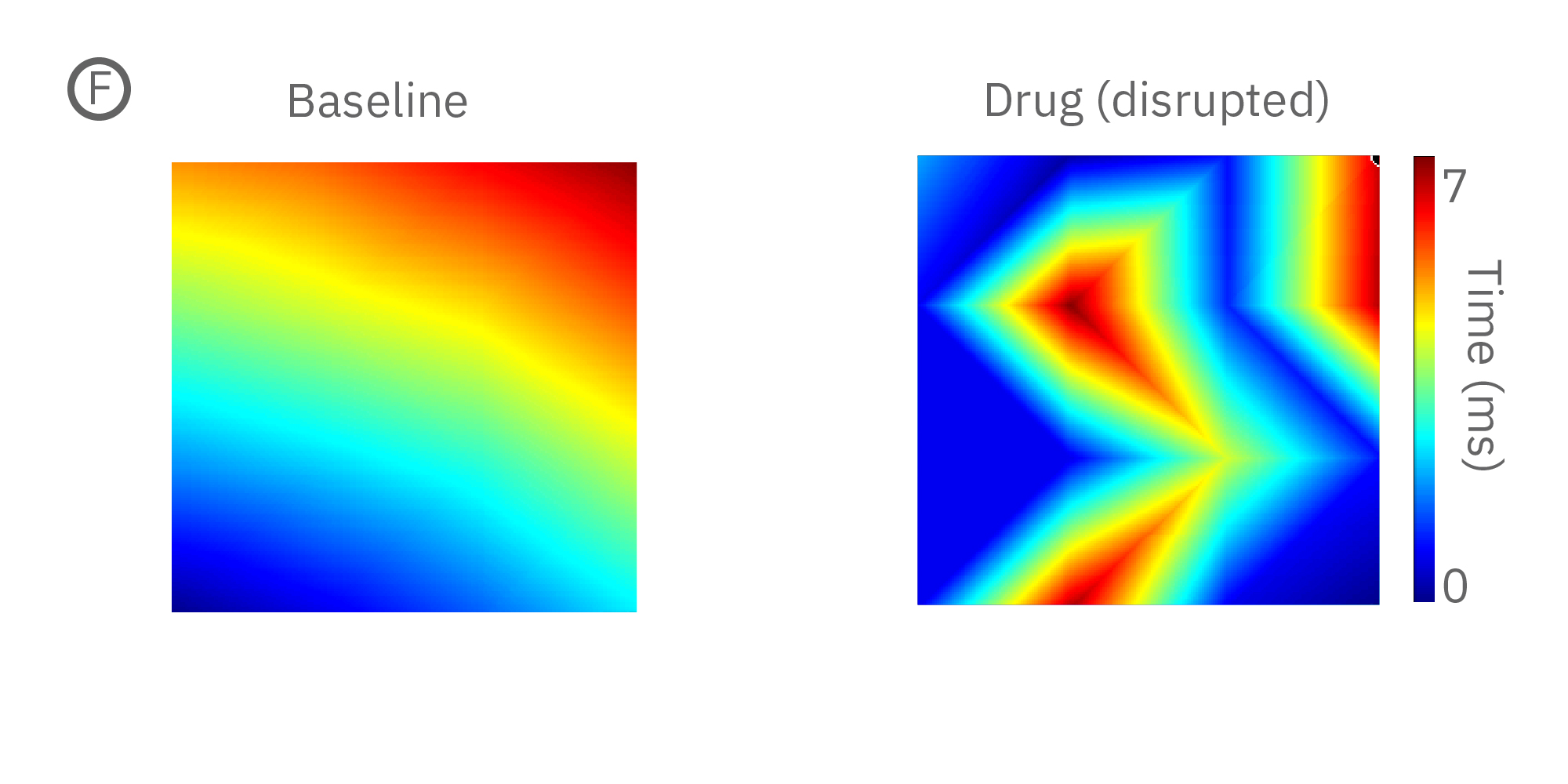
(F) 薬剤による伝播パターンの不規則化の例(左>中央)。右図は薬剤投与によるビート毎の伝搬規則性の減少を示す。
同一電極を用いた構造的・機能的心毒性の検証
心毒性は心臓の機能のみならずその構造にも影響を及ぼします。Maestro Pro/Edgeでは、MEA測定による心筋細胞の電気的な活動の検出に加え、同電極を用いて細胞の生存率(電極への被覆によるインピーダンス変化)を測定することが可能です。同一サンプルからの両測定により多くの情報が得られます。また、非侵襲電極による測定は、数日間に渡る慢性評価にも最適です。
Learn more about MEA Viability.
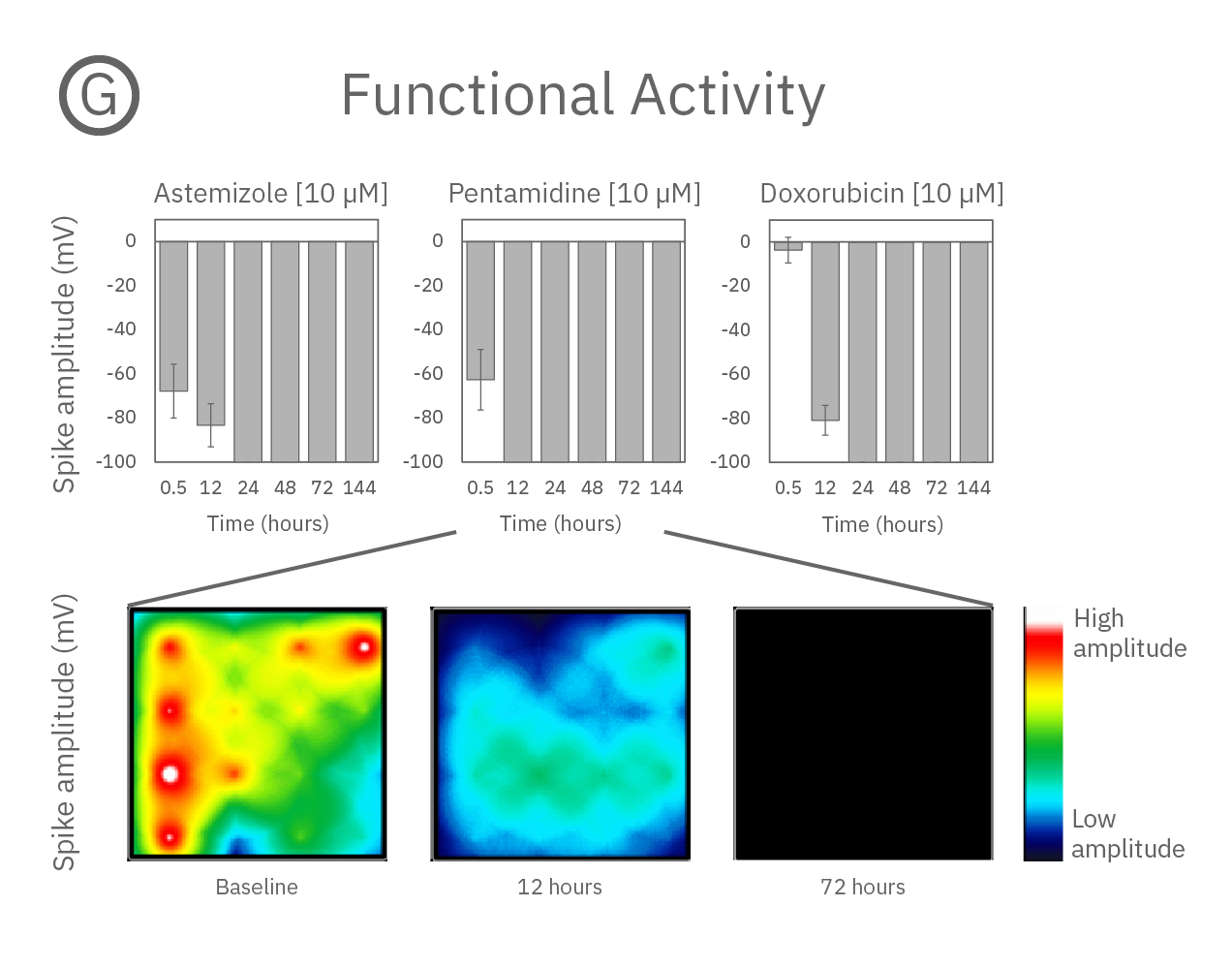
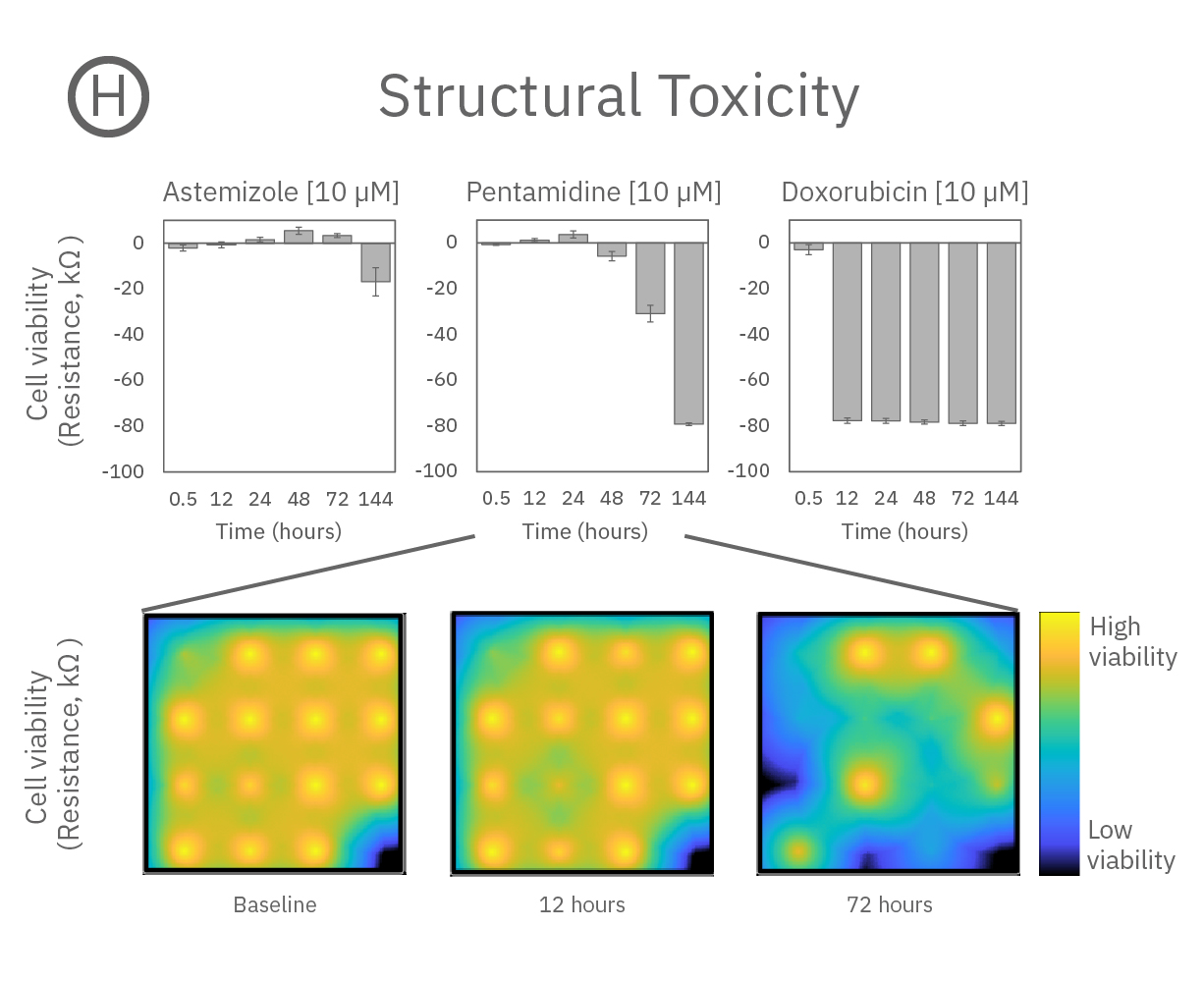
心機能に影響を及ぼす3種の薬剤 (Astemizole、Pentamidine、Doxorubicin) 投与による経時的な変化を検証した。
(G) 細胞外電位測定によるスパイク振幅値の経時的変化を示す。3種類の薬剤にて、時間の経過と共に振幅値は減少及び消失し、強い心毒性が示唆された。
(H) Viability (インピーダンス)の経時的変化を示す。強い毒性は、Pentamidine (hERG trafficking 阻害剤) とDoxorubicin(抗がん剤)のみに見られた。また、細胞死に至る速さは薬剤毎に異なり、Doxorubicinでは24時間以内であった一方、pentamidineでは6日間を要した。
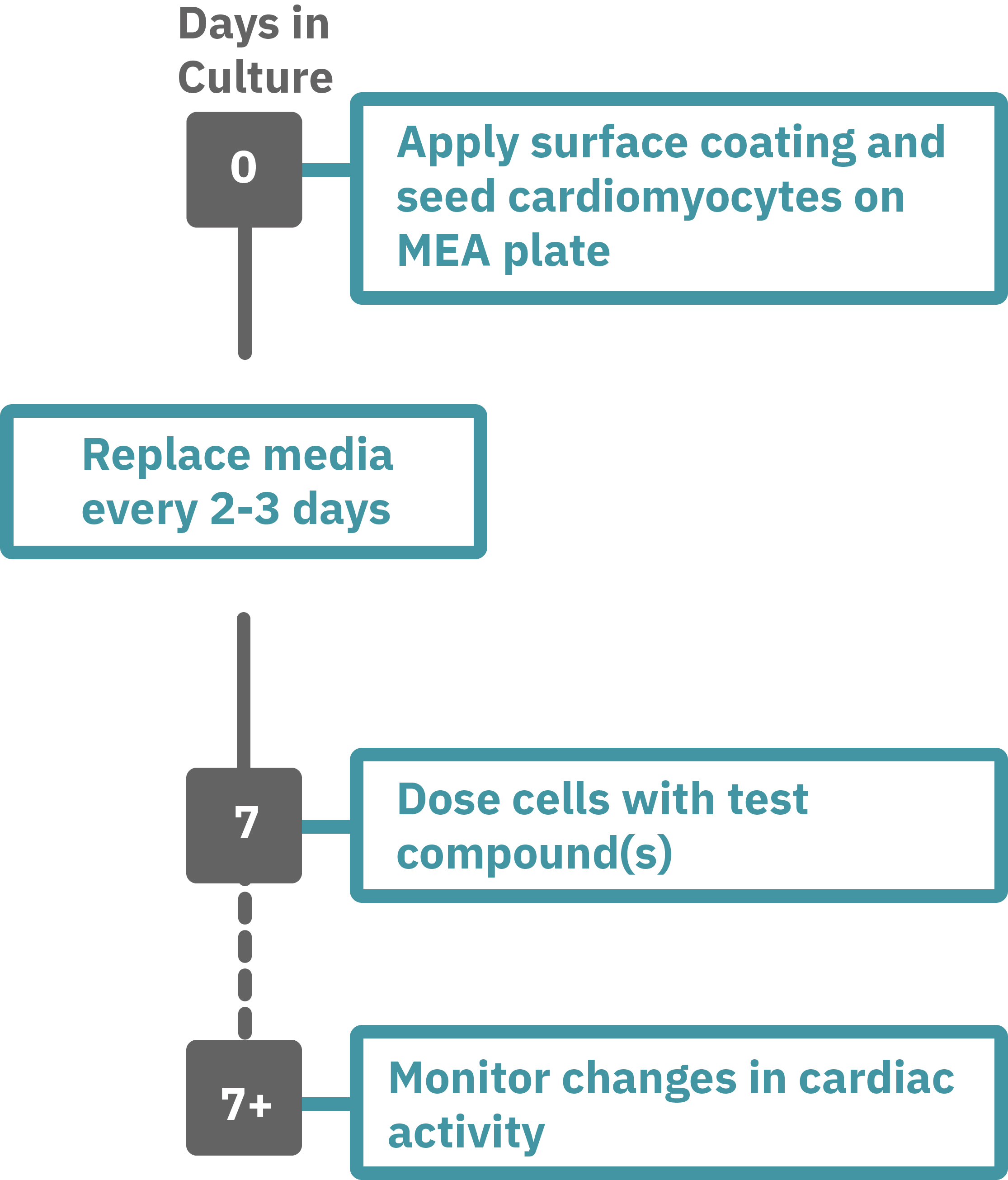
Maestro Pro/Edge による心毒性評価は非常に簡単です。
コーティングされたMEAプレート上に直接心筋細胞を播種します(Day 0)。2-3日毎に培地交換をしながら、一定期間細胞を培養します。
データ測定日 (例: Day 7+) にプレートを Maestro に搭載し測定を行います。データは付属のソフトで解析可能です。
Maestro MEA による心毒性・安全性評価:特徴
-
1システムで4種類のアッセイ - Maestro Pro/Edgeでは、1枚のプレートで次の4種類のアッセイが行えます。[1] 細胞外電位応答 [2] シグナル伝播 [3] Contractility (弛緩収縮評価) [4] LEAP (活動電位形態シグナル)
-
ラベルフリー・リアルタイムで細胞の電気的な活動を測定 - プレート上の電極を用いて心筋細胞の活動電位を測定します。ラベルフリー、リアルタイムな測定は、試薬など2次的要因によるゆがみがなく、細胞の変化をより正確にとらえます。また、数日から数週間に渡る慢性評価にも最適です。
-
同一プレート上で培養から測定まで - MEAプレート上で直接細胞を培養し、測定します。環境要因による変化を最小限にとどめながら、細胞への負担が少ない状態で、細胞の変化を検出することができます。
-
安定した環境下での実験 – 温度・CO₂ 濃度は、装置搭載のコントローラで自動制御されます。また、Maestro は外来ノイズ・振動に影響されにくい設計になっています。安定した環境で毎日安心して実験に望めます。
-
簡単操作 - 電気生理未経験の方でも簡単に実験が行えます。MEAプレート上に細胞を培養し、装置に搭載するだけで、心筋細胞の電気的な活動の測定が可能です。付属のソフトウエアパッケージを用いて、数クリックで複数の指標に基づいた解析結果が得られ、結果の作表まで行うことができます。

Cardiac LEAP
Show Full DetailsWhat is LEAP?
LEAP technology enables noninvasive, label-free monitoring of the cardiac action potential in a high-throughput, real-time format. LEAP can be used for quantification of action potential morphology, repolarization irregularities such early after depolarizations (EADs), and arrhythmic risk factors such as triangulation.
Why LEAP matters?
The LEAP signal accurately reflects the shape and duration of the underlying action potential. The large signal allows for automated detection and classification of arrhythmic events, such as notched EADs, rolling EADs, or ectopic beats. LEAP also provides metrics not available from the field potential, such as rise time and triangulation.

Cardiac MEA
Show Full DetailsWhat is Cardiac MEA
Cardiac MEA (microelectrode array) technology is a label-free method for recording electrical activity in heart cells. Tiny electrodes embedded in the culture surface detect electrical signals from cardiomyocytes, allowing continuous, noninvasive monitoring of cardiac function in real time. This technology provides insights into cardiomyocyte function, rhythm, and drug response in 2D and 3D cell models
Why Cardiac MEA matters
Traditional cardiac assays rely on indirect, low resolution, or endpoint measurements that fail to capture electrical function, rhythm changes, and time-dependent drug effects. Cardiac MEA technology addresses this gap by providing direct, physiologically relevant measurements of cardiac electrophysiology, supporting safer drug development, improved cardiotoxicity screening, and more predictive disease models.