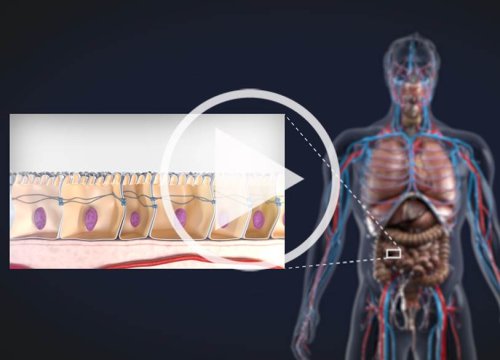
ヒトの体内において、細胞のバリアは多くの表層を形成し、組織を保護したり体内への物質の出入りをコントロールしています。これらの細胞バリアは、主に内皮細胞と上皮細胞で構成されており、バリアの浸透機能は、炎症、感染症、がん細胞の転移、白血球遊走などの生物学的事象によって改変されます。
Axion BioSystems の Maestro では、インピーダンス変化の検出により、細胞の変化をラベルフリーで連続して、測定・解析することが可能です。数日間に渡るバリアの破綻を連続して追跡することが可能です。
生体バリア完全性評価
経上皮電気抵抗 (TEER) はバリア機能の評価にしばしば用いられます。
Maestroによるインピーダンスアッセイは、経上皮電気抵抗値を測定し、バリアインデックスとして指標化します。Maestroは、複数の周波数条件でインピーダンスを測定します。低周波下で得られるインピーダンス値を、細胞の電極への被覆を示唆する高周波下のインピーダンス値でノーマライズすることにより、微細なバリア機能の変化を捉えます。
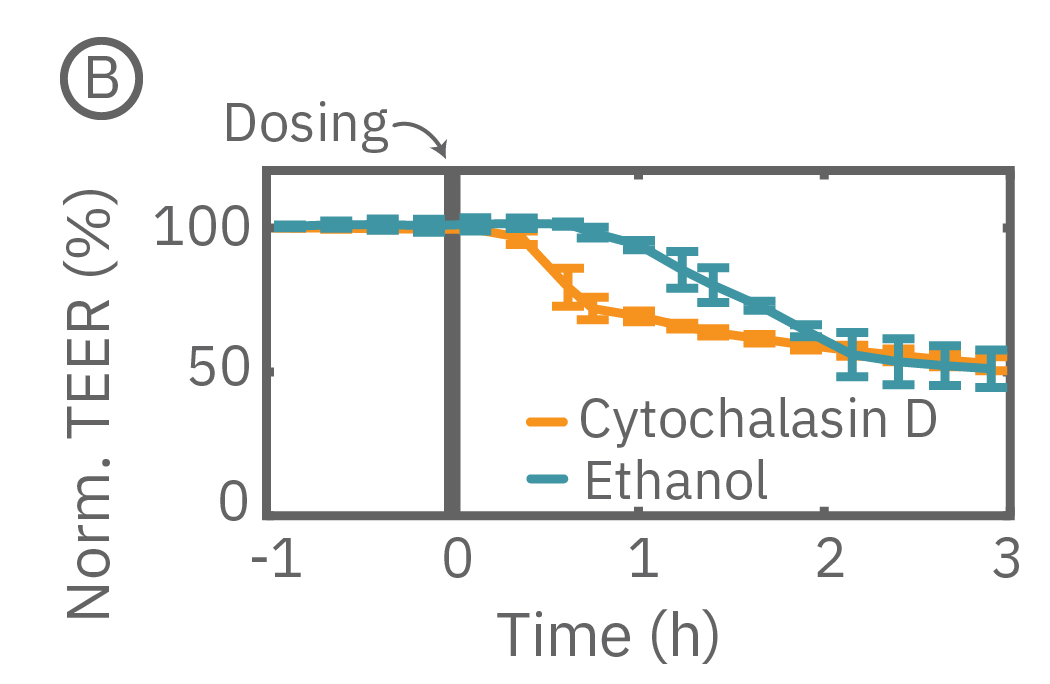
バリアの浸透機能は、一般的な薬剤や分子によっても改変されることがあります。本事例では、96 well フォーマットの CytoView-Z プレート上に Calu-3 細胞を播種し、Maestro Z で14日間に渡り連続してTEERを測定しました。細胞がプレート上でコンフル状態になった後、サイトカラシンD、エタノールをそれぞれ投与しました。サイトカラシンDは、アクチン重合を阻害し、タイトジャンクションの漏洩を増加させますが、この現象が急激なTEERの減少(B図オレンジ色)にて示されました。一方、エタノールは、タイトジャンクションにおける2種類の重要なたんぱく質であるZO-1とクローディン1結合に比較的ゆっくり影響を及ばしますが、この現象が緩慢なインピーダンスの減少によって示されました(B図水色)。
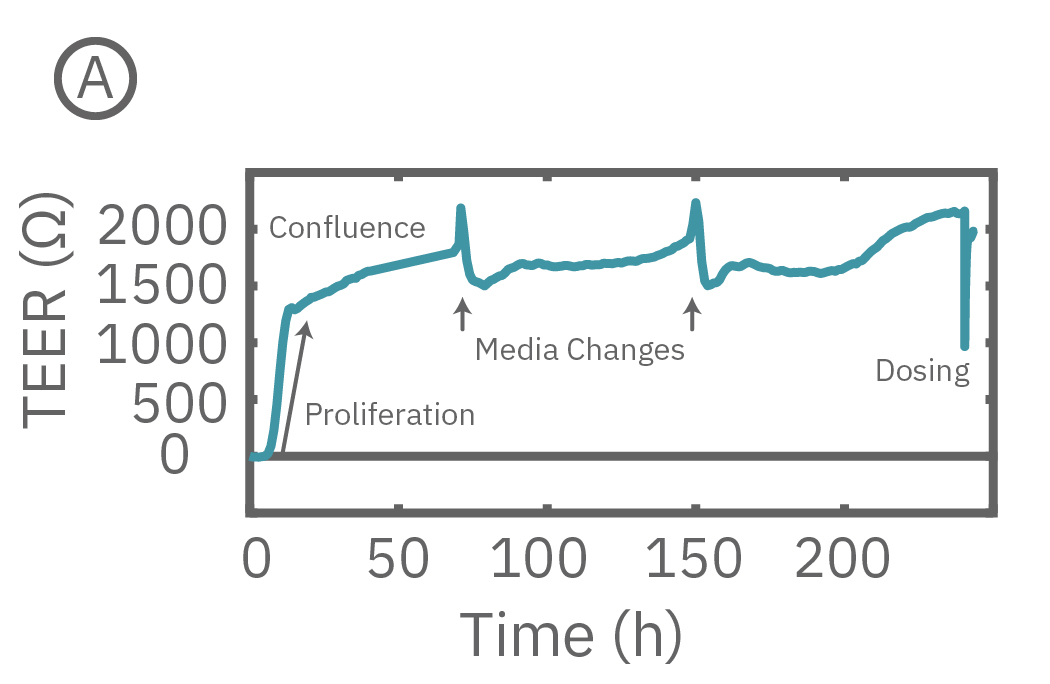
(A) CytoView-Z プレート上に Calu-3 細胞を播種し14日間に渡り連続してTEERを測定した。
(B) 14日後にサイトカラシンD (オレンジ色)とエタノール(水色)をプレート上の異なる well に投与したところ、TEERの減少が得られた。
Disease-in-a-dish モデルでのバリア機能評価
バリア機能はヒトの体内において重要な役割を担っていますが、嚢胞性線維症 などの病気により破壊することもあります。Calu-3細胞株は、不死化細胞であり、タイトジャンクションを形成します。また、嚢胞性線維症膜コンダクタンス制御因子 (CFTR) を発現させ、βアドレナリン性の刺激によりイオンを透過させます [Shen et al., 1994]。本事例では、TEER アッセイによりCFTRのこれらの機能を検証しています。
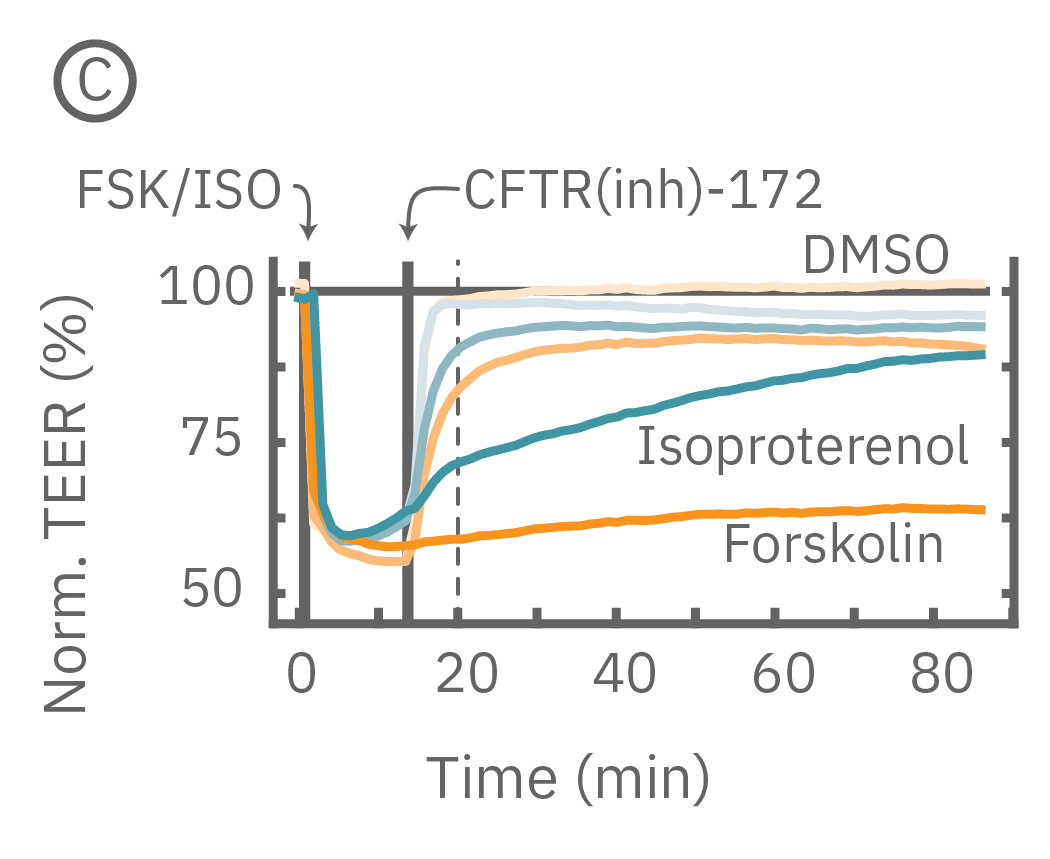
(C) プレート上で培養されたCalu-3細胞に、イソプレテレノール (水色、100 μM)とフォルスコリン (オレンジ色、10 μM)を投与したところ、TEERが急激に減少した。続いてCFTR(inh)-172を30 μM 及び300 μM の濃度で添加したところ、TEERに回復がみられその上昇は濃度に依存した。
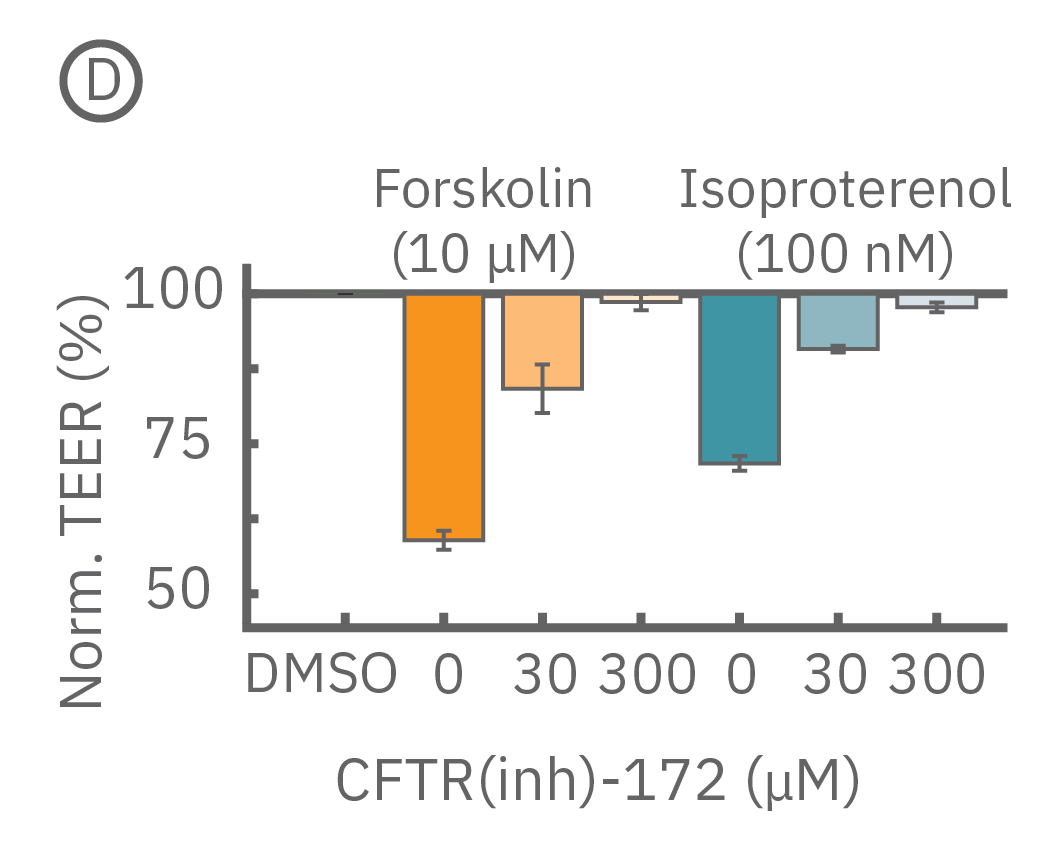
(D) CFTR(inh)-172投与から5分後 (C図点線箇所) のTEER。フォルスコリン、イソプレテレノール共に、CFTR(inh)-172による抑制反応は、濃度に依存した。
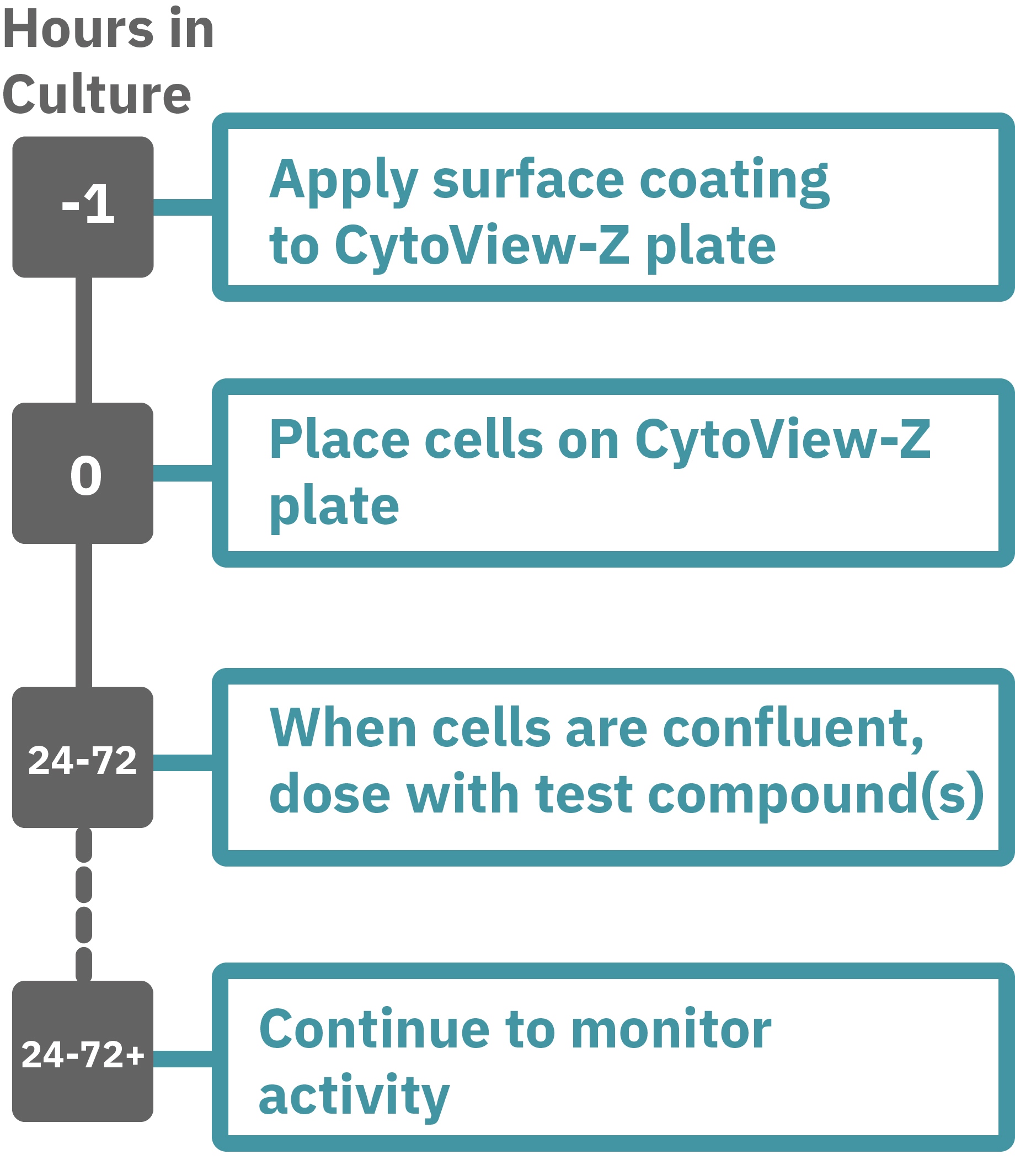
Maestroによる、バリア機能評価はとても簡単です。事前コーティングされた CytoView-Zプレート上に細胞を播種します (Hour 0)。Maestroシステムにプレートを搭載すると同時に、温度・CO₂ 濃度制御とインピーダンス測定が開始されます。細胞の増殖・電極への接着に伴いインピーダンスが上昇します (Hour 0 ~ 24-72)。
細胞がコンフルな状態なったら化合物などを投与し、以降数日間に渡って生体バリアの変化をラベルフリーで測定します。

Maestro Z/ ZHT, Pro/EdgeによるTEERアッセイ:特徴
-
経時的観察 - 96或いは384 well 同時に測定します。細胞バリア、タイトジャンクションの変化を連続して測定し、専用アプリで、実験室の外からでもライブデータの確認が可能です。
-
ラベルフリー - 平面電極によるインピーダンス測定は、染色・試薬などを必要としません。ラベルフリー測定で数日間に渡る測定・観察が可能です。
-
インキュベータ不要 - Maestro には温度・CO2濃度コントローラが内蔵されています。インキュベータ等の周辺装置は不要。安定した環境下で数日間に渡る連続測定が可能です。
-
細胞可視 - CytoView-Z 96 well プレート底面中央部は透明になっています。必要に応じて、細胞の観察が可能です。
-
培養から測定まで同一プレート使用 - アッセイの全行程を同一プレートで行います。他のハイスループット・プラットフォーム(例:フローサイトメータ)のような容器の入れ替えなどは不要。細胞への負担を最小限に抑えることができます。
-
スマートフォン・アプリ - 専用のスマートフォンアプリに対応しています。数日間に渡るバリアの変化の様子を、実験室の外からでも、リアルタイムに観察して頂けます。
-
簡単 - セミ・オートメーションシステムです。ハードウエアの操作はボタン1つ。専用のソフトは、インピーダンスの変化をリアルタイムで表示します。解析結果のエクスポートも容易です。
TEER
Show Full Details
TEER Assay: Barrier function on the Maestro Z
Endothelial and epithelial cells form barriers throughout the body that serve to protect and compartmentalize, regulating what gets in and out of the tissue. Endothelial cells line the inside surfaces of the body, such as the inner layer of blood vessels, while epithelial cells line the outside surfaces, such as skin and most organs. Both cell types express tight junctions, allowing them to link tightly with neighboring cells to form a selectively permeable barrier.
These important barriers can be disrupted by disease, injury, infection, or drugs. Thus, measuring barrier integrity and permeability is vital for understanding disease and predicting drug behavior.
The Maestro Z can continuously monitor barrier integrity via measurements of electrical resistance across the cell layer, a technique known as transepithelial or transendothelial electrical resistance (TEER).
How does TEER work on the Maestro Z?
The Maestro Z uses impedance technology to measure TEER, which is used to assess the barrier integrity of cells. TEER quantifies how much of an electrical signal is blocked by a cell layer when a small AC current is passed from one electrode to another. Since impedance is noninvasive and label free, barrier integrity can be continuously monitored for minutes, hours, or even days without disturbing the cellular biology.
Traditionally, TEER is measured by placing two chopstick-style electrodes on either side of a transwell insert with a confluent cell layer. Manual methods, where the electrodes are placed well-by-well, are highly labor intensive. Even automated TEER methods are typically constrained to lower throughputs, like 24-well plates. In contrast, TEER on the Maestro Z is high throughput and hands free, allowing measurements across 96 wells simultaneously.
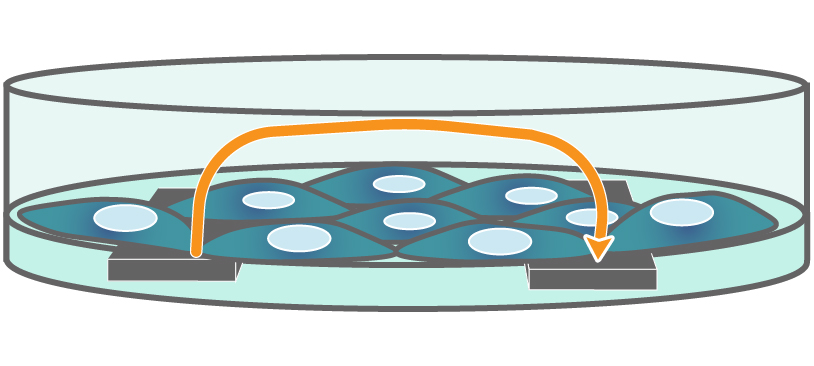
TEER on Maestro Z. Electrodes embedded in the cell culture substrate at the bottom of each well detect small changes in the impedance of current flow. Barriers, such as tight junctions between cells, resist current flow, leading to an increase in TEER measurements of barrier function.
Measure coverage and TEER in the same assay
Traditional TEER measurements require a fully confluent cell layer to accurately measure barrier function. In contrast, the Maestro Z tracks coverage and TEER simultaneously and continuously by measuring impedance at multiple frequencies.
Measuring impedance at low frequency is highly sensitive to the intercellular barrier formed by tight junctions and the paracellular barrier formed by cell membranes. On the other hand, measurements at higher frequency can be used to quantify coverage of the well bottom. In other words, low frequencies are sensitive to “what” cells are there, whereas high frequencies are sensitive to “how many” cells are there. By calculating the ratio of resistance at these two frequencies, the Impedance Module can normalize TEER to the cell coverage and give the result as barrier index.
In this example, Maestro Z resistance measurements were used to continuously and simultaneously monitor cell coverage and barrier function of two human lung epithelial cell lines. Both cell lines reach full coverage within 24 hours. However, only the Calu-3 cells, which express tight junctions, produce a significant low-frequency TEER signal, indicating a strong cellular barrier
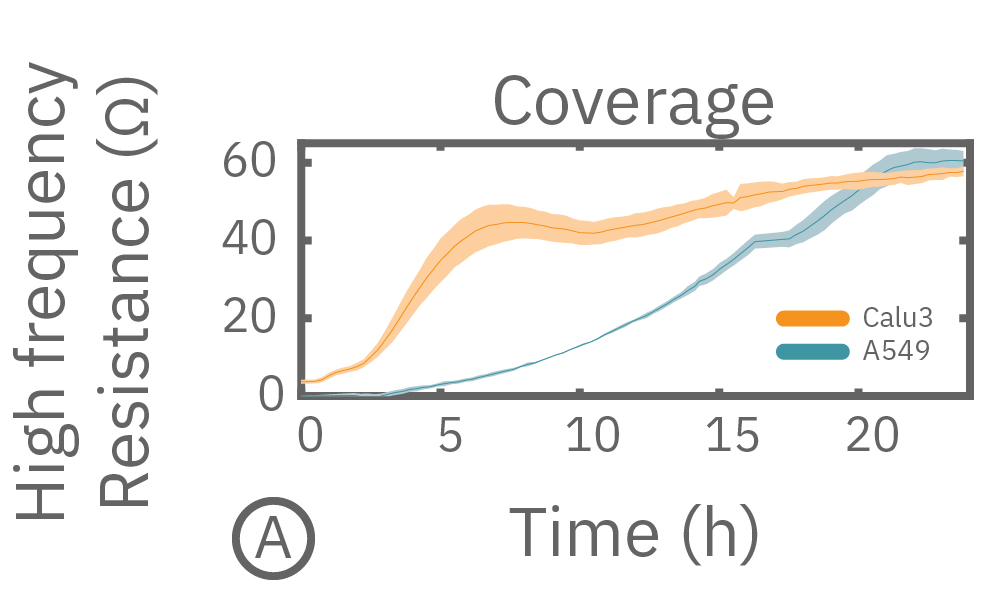
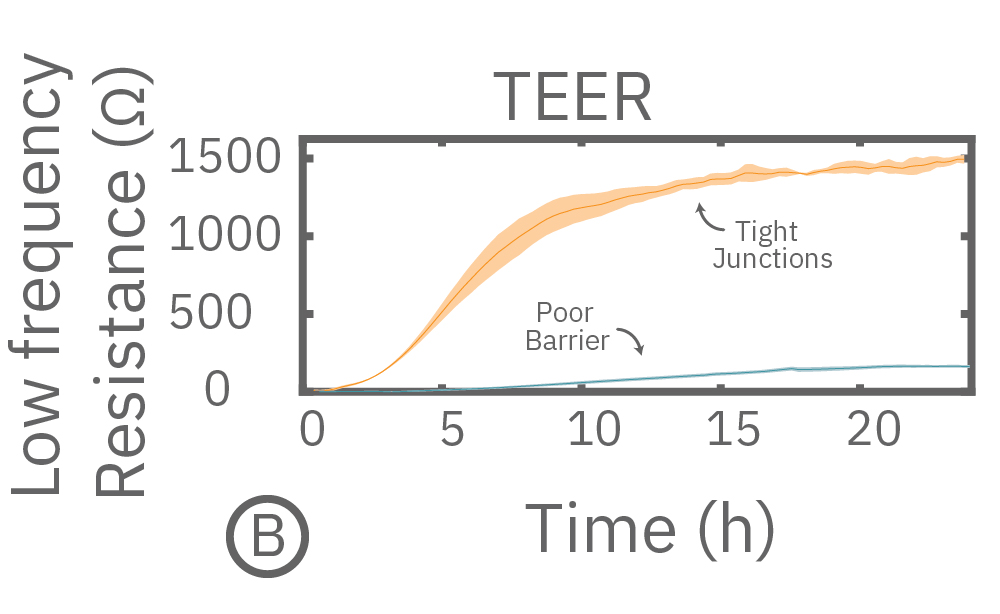
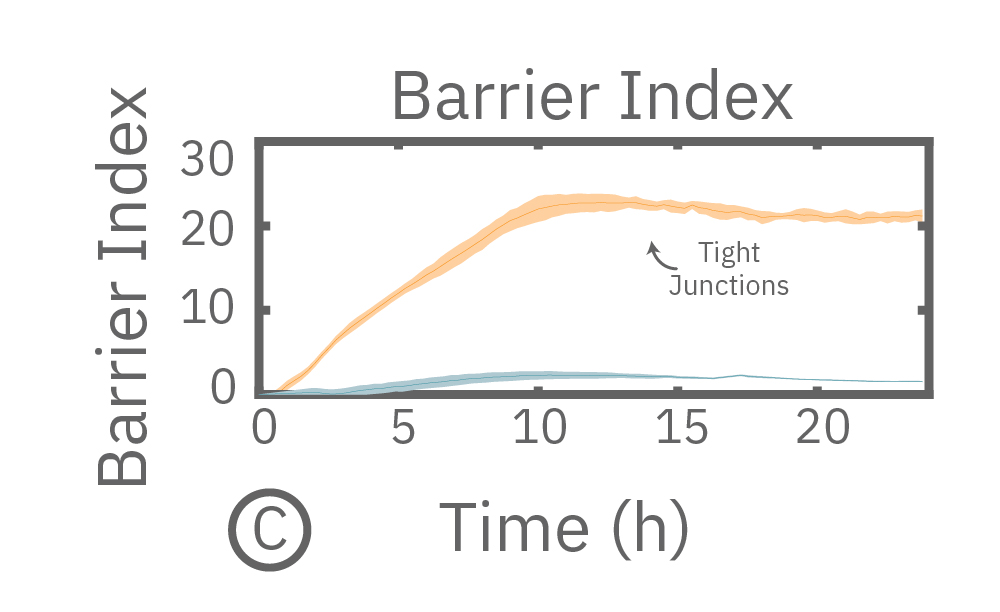
(A) Coverage, measured as resistance at 41.5 kHz, increases over time for both Calu-3 and A549 cells. (B) TEER, measured at 1 kHz, reveals that only Calu-3 cells form a strong barrier though, as they express tight junctions to block flow between neighboring cells. (C) Barrier Index both reveal that only Calu-3 cells form a strong barrier though, as they express tight junctions to block flow between neighboring cells.
Real-time detection of rapid barrier disruption
In addition to long-term monitoring, TEER on the Maestro Z is sensitive to small, transient disruptions to barrier function. Many signaling molecules in the body can alter barrier permeability. TEER on the Maestro Z can capture these disruptions in real time without being limited to a single endpoint measurement.
Here, TEER was monitored continuously for Calu-3 cells in a 96-well CytoView-Z 96-well plate on the Maestro Z. After reaching confluence, cells were dosed with cytochalasin D and ethanol. Cytochalasin D inhibits actin polymerization to increase tight junction permeability, as reflected by the rapid decrease in TEER. Ethanol acts more slowly on ZO-1 and Claudin-1, two key tight junction proteins. The rapid disruption, as well as the mechanistically distinct dynamics, are captured by TEER measurements on the Maestro Z.
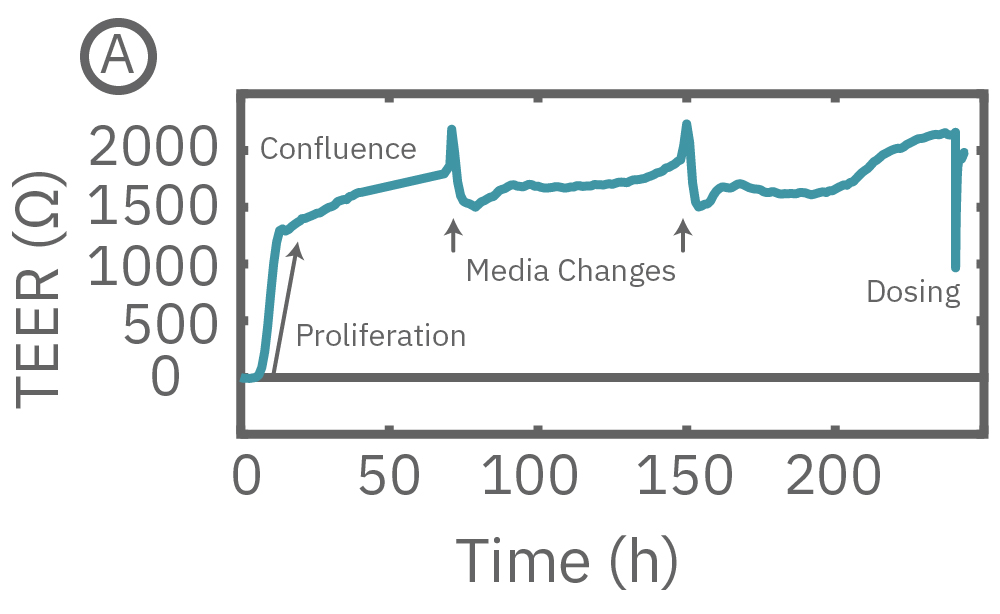
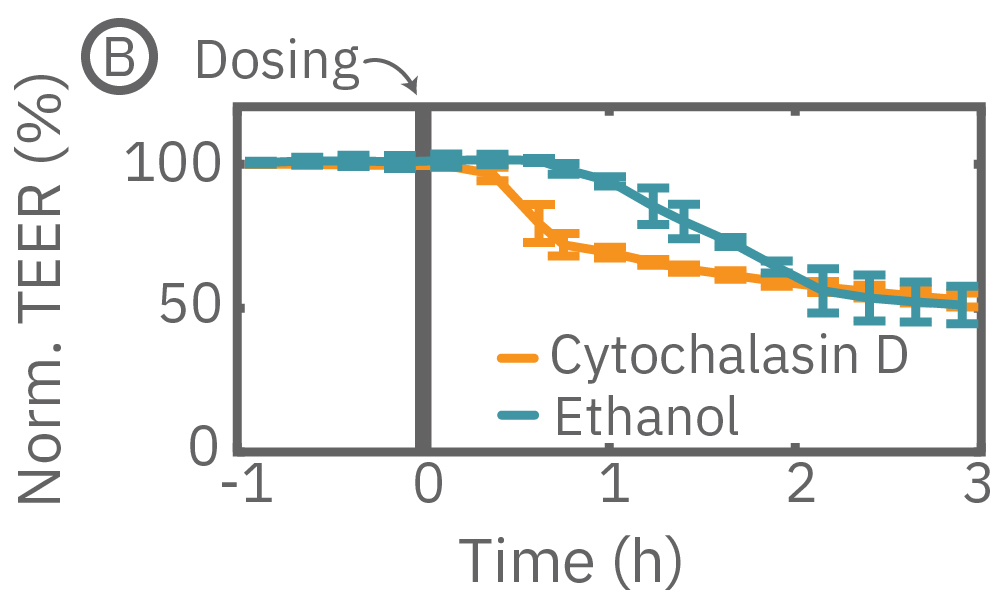
(A) TEER can be measured over the course of proliferation and barrier formation. (B) TEER is highly sensitive to transient drug-induced changes in barrier permeability, such as those induced by cytochalasin D and ethanol.

Impedance
Show Full DetailsImpedance: For real-time cell analysis
Impedance-based cell analysis is a well-established technique for monitoring the presence, morphology, and behavior of cells in culture. Impedance describes the obstruction to alternating current flow. To measure impedance, small electrical currents are delivered to electrodes embedded in a cell culture substrate. The opposition to current flow from one electrode to another defines the impedance of the cell-electrode interface. When cells are present and attached to the substrate, they block these electrical currents and are detected as an increase in impedance.
Impedance is sensitive to many aspects of cell behavior: attachment, spreading, shape, cell-cell connections (e.g. tight junctions), and death. Even small transient changes, such as swelling or signaling, are detectable by impedance. Because impedance is noninvasive and label free, the dynamics of these changes can be monitored in real time over minutes, hours, or even days without disturbing the biology.
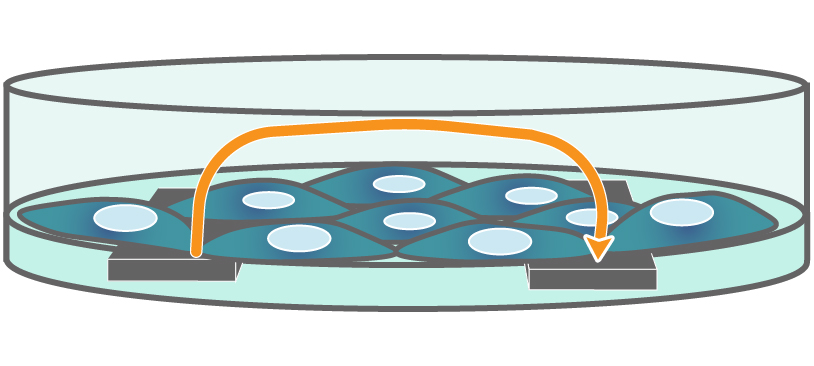
Interdigitated electrodes embedded in the cell culture substrate at the bottom of each well detect small changes in the impedance of current flow caused by cell presence, attachment, and behavior.
In the example below, the electrodes are initially uncovered before cells are added. The electrical current passes easily and the impedance is low. When cells begin to attach and cover the electrodes, less electrical current passes and the impedance is high. After dosing with a cytotoxic agent, cells die or detach, and the impedance decreases back towards baseline.
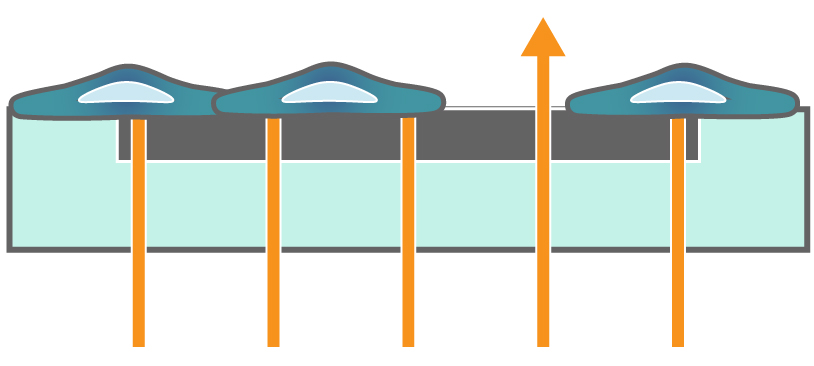
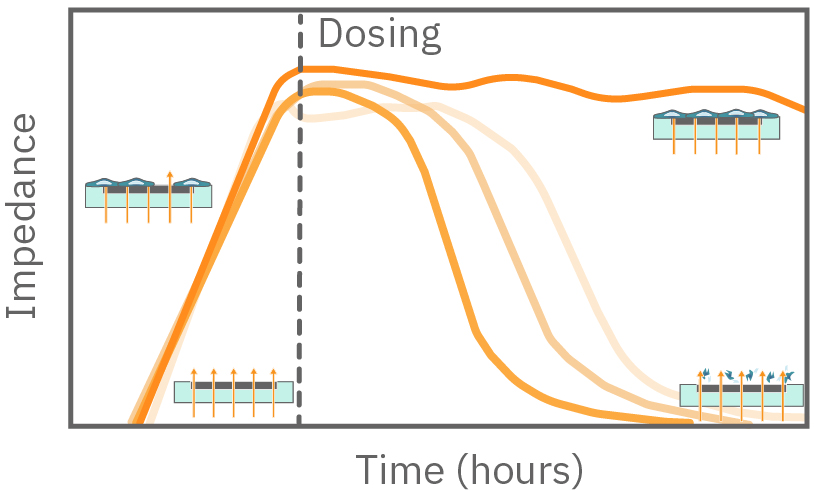
Impedance measures how much electrical signal (orange arrows) is blocked by the cell-electrode interface. Impedance increases as cells cover the electrode and decreases back to baseline due to cell death.
Continuous cell monitoring
Many cell-based assays are endpoint assays, limited to a single snapshot in time. Repeating these assays at multiple time points can be labor intensive, time consuming, and costly. Key time points can be easily missed. Impedance-based cell analysis is nondestructive and label free, meaning that cellular dynamics can be monitored continuously.
The impedance assay can be used to characterize dynamic cell profiles, revealing how cells grow, attach, and interact over time. Each cell type exhibits a different cell profile, or “fingerprint”, of dynamic cell behavior. These profiles are sensitive to cell type, density, purity, and environmental factors. In this example, the Maestro Z impedance assay readily distinguished cell profiles across different cell densities and cell types.
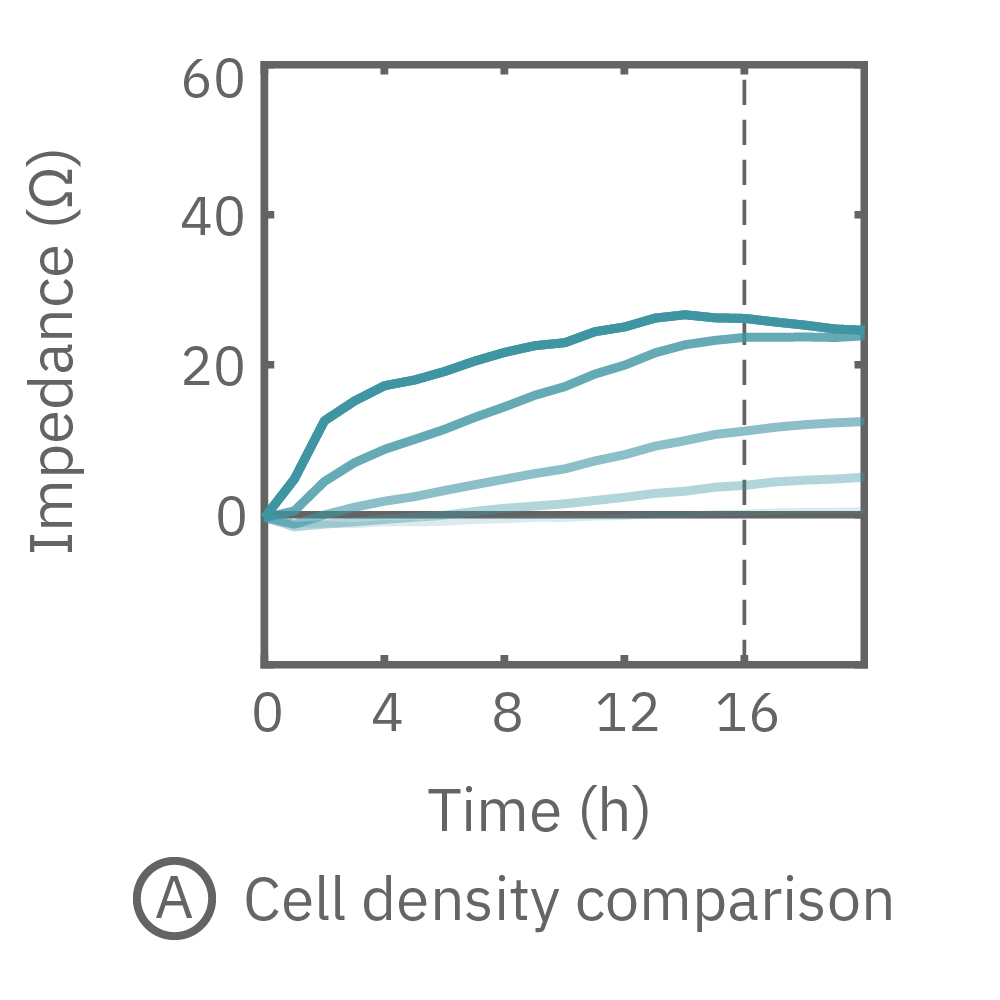
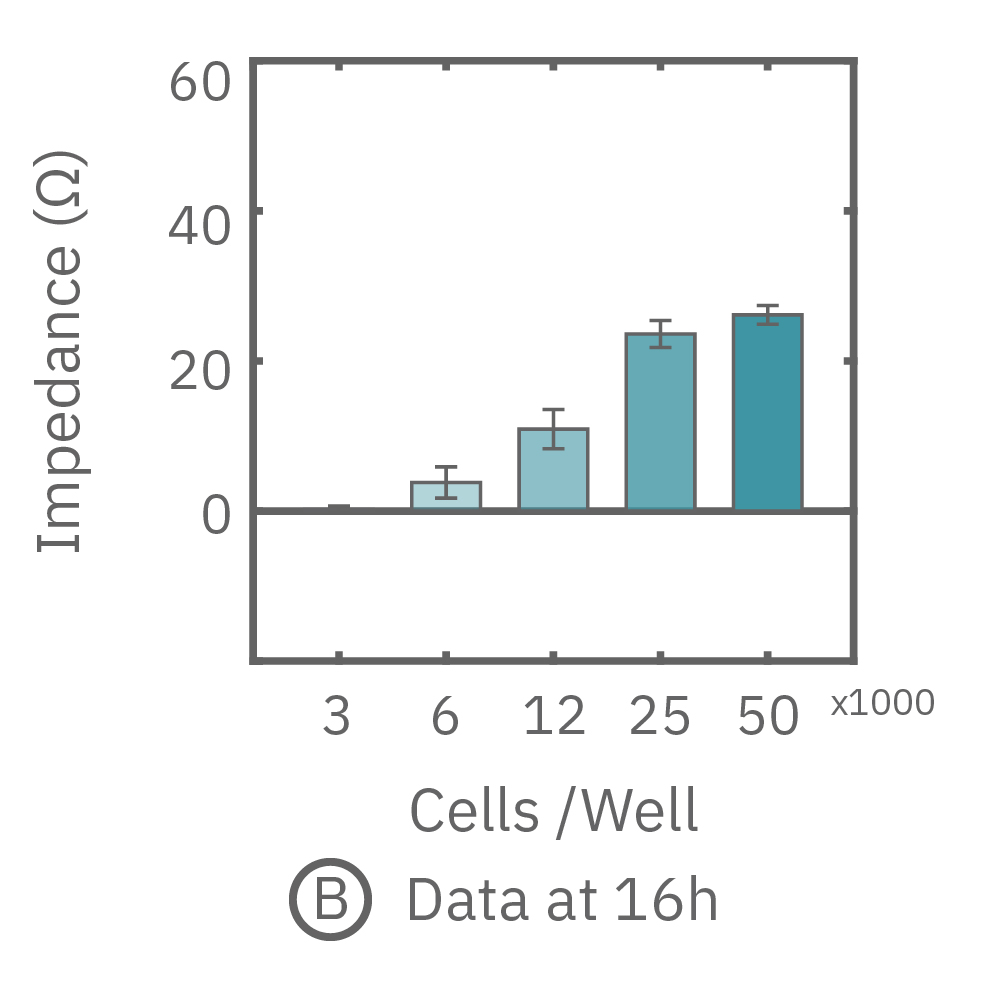
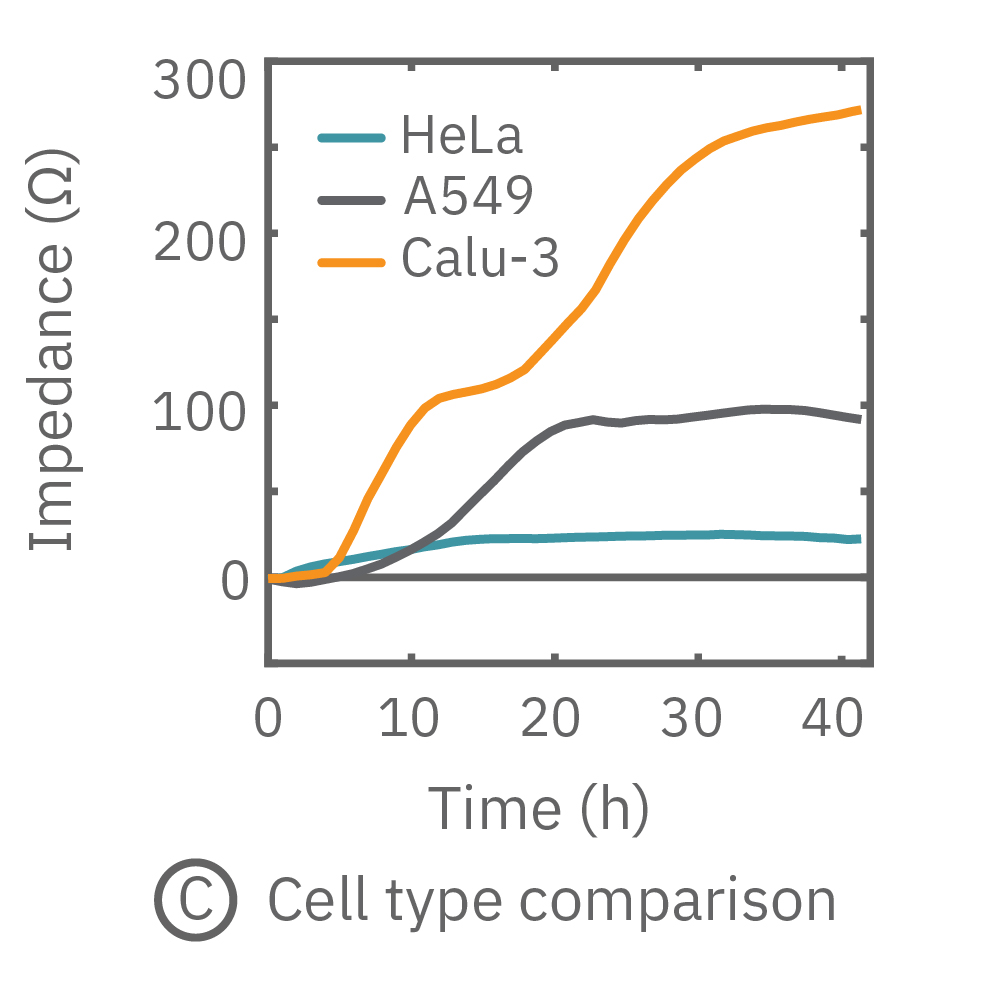
(A, B) HeLa cells were seeded on a CytoView-Z plate at varying densities and the impedance was continuously monitored by the Maestro Z. Impedance scaled proportionally with cell density and readily distinguished different densities of the same cell type. (C) Maestro monitored the growth of three cell types, HeLa, A549, and Calu-3, and readily distinguishes their distinct cell profiles over time.
The Maestro Z impedance assay can also be used to capture the kinetics of cell responses to drugs or immune cell therapies. The kinetics, which cannot be captured by endpoint assays, often provide key insights into the efficacy of novel therapies. In the example below, the Maestro Z impedance assay was used to quantify the kinetics of cytotoxicity of chemotherapy agents.
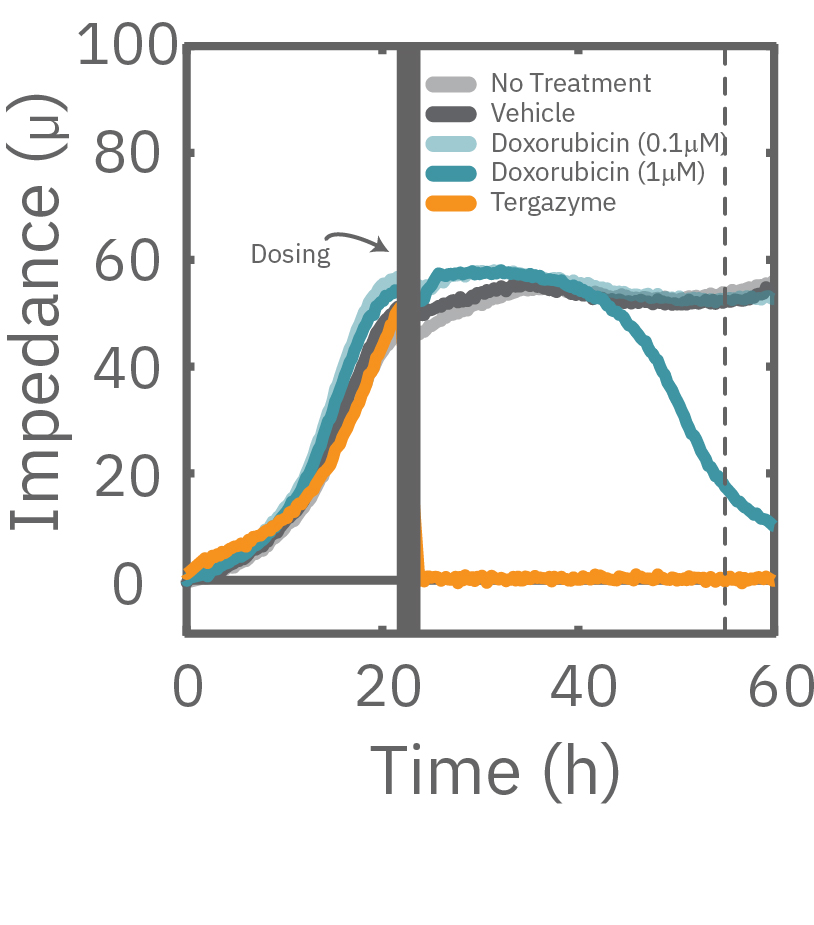
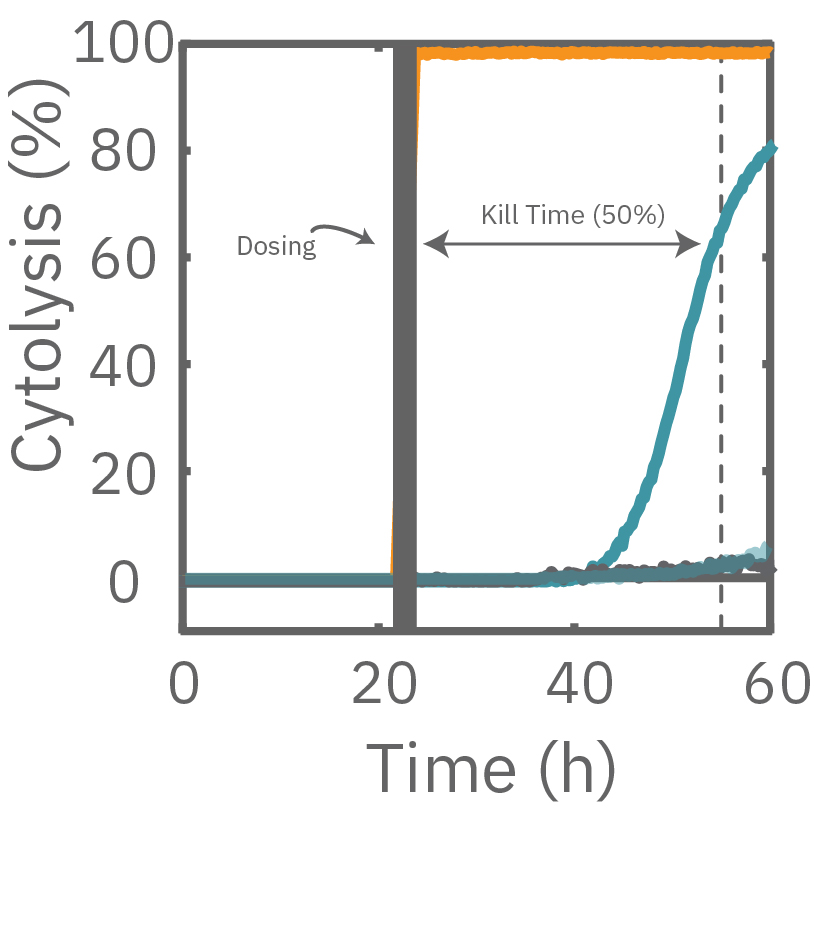
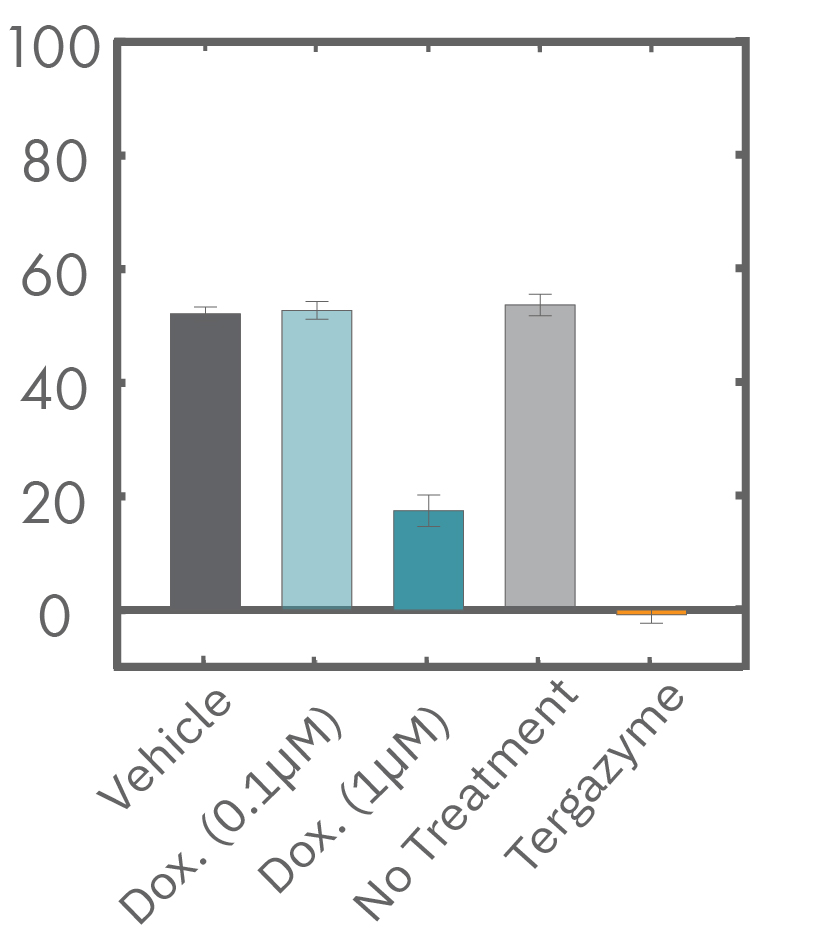
A549 cells were dosed with dox, vehicle (DMSO), or tergazyme. Wells dosed with tergazyme showed an immediate decrease in impedance, reflecting complete cell death. Higher doses of dox resulted in a slower decrease in impedance and cell death. Cells dosed with 1 μM dox reached 50% cytolysis at 31 hrs.
Different frequencies reveal cell properties
Impedance varies with frequency, such that different frequencies reveal different aspects of cell biology. The small currents used to measure impedance will always take the path of least resistance. At low frequencies, such as 1 kHz, the impedance of the cell membrane is relatively high, forcing the current to flow under and between the cells. Low frequencies provide details about barrier integrity, the presence of gap junctions, and transepithelial or transendothelial resistance (TEER).
At high frequencies, such as 41.5 kHz, the impedance (and capacitive reactance) of the cell membrane is relatively low. Thus, most of the current couples capacitively through the cell membranes, providing information about the cell layer such as confluency and coverage.
In other words, low frequencies are sensitive to “what” cells are there, whereas high frequencies are sensitive to “how many” cells are there. The Maestro Z impedance assay uses multiple frequencies to provide the most information about the cells, simultaneously, continuously, and in real time.
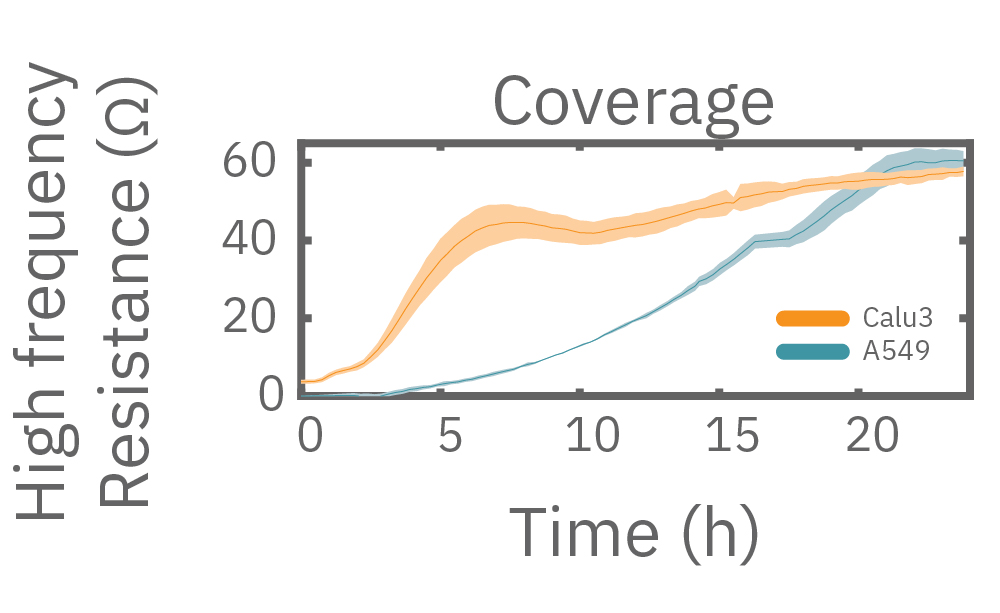
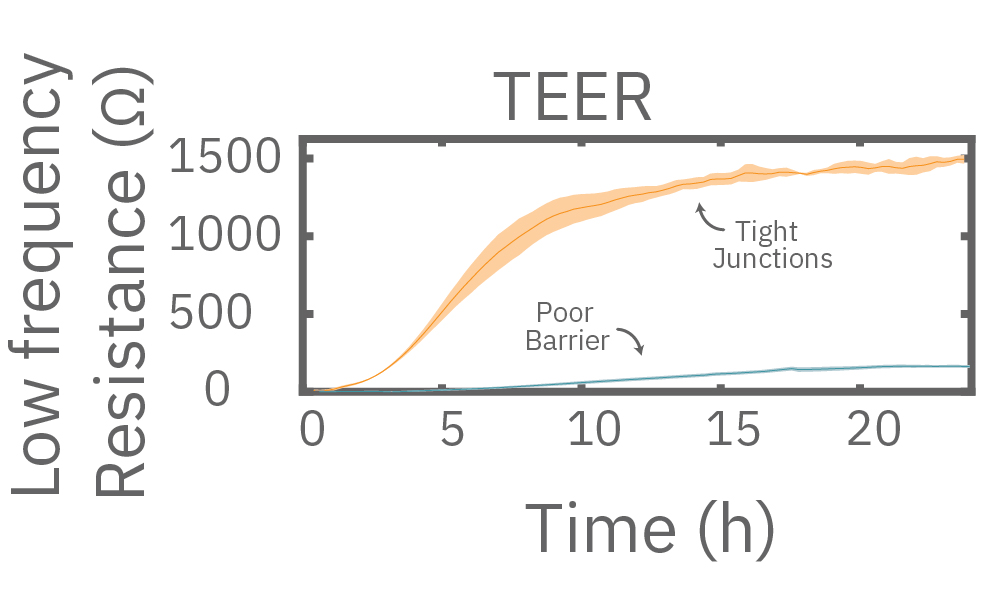
Multiple frequencies were used to simultaneously and continuously monitor the coverage and barrier function (TEER) of Calu-3 and A549 cells. Coverage, measured as resistance at 41.5 kHz, increases over time for both cell types. TEER, measured at 1 kHz, reveals that only Calu-3 cells form a strong barrier, as they express tight junctions to block flow between neighboring cells.